The oxygen reduction reaction (ORR) is a key process in electrochemical energy conversion and storage devices such as fuel cells and metal-air batteries. Platinum (Pt)-based catalysts are generally used in ORR, but there are unsurmountable problems such as scarcity, high cost, and long-term stability/deterioration associated with performance degradation during operation as well as poisoning by methanol fuel.
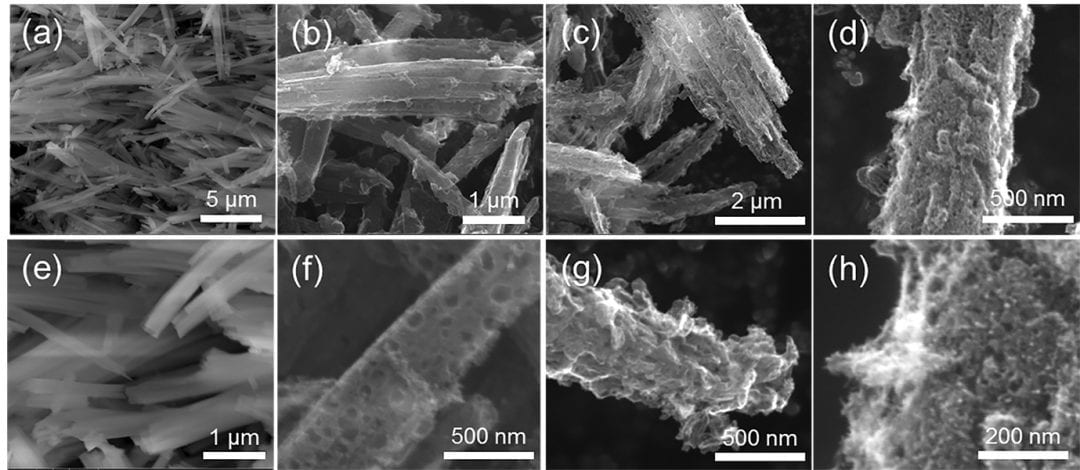
Among the candidates to replace platinum, carbon catalysts doped with nitrogen and transition metals exhibit the highest ORR performance. To date, metal-organic frameworks (MOF)-based carbon constitutes a promising platform due to the porous structure and the uniform distribution of nitrogen and transition metals, resulting in a number of active sites composed of metal-nitrogen-carbon. However, the lack of nanostructure engineering can induce aggregation of MOF nanocrystals during the annealing treatment at temperatures above 800 °C to form micrometer-sized agglomerates.
Professor Arumugam Manthiram and his research group at the University of Texas at Austin have succeeded in producing a highly efficient one-dimensional ORR carbon catalyst via a MOF-derived approach. The carbon catalyst doped with nitrogen and cobalt has a one-dimensional structure composed of hollow spheres and nanotubes. A self-template conversion technique used in this study replaces the conventional template-based synthesis method to provide excellent additional functionalities and prevent aggregation during the sintering of the MOF nanocrystals.
In the electrochemical measurements, the Co-N-C one-dimensional carbon catalyst showed higher activity than the conventional Pt/C catalyst in alkaline electrolyte. The presence of chemically stable Co-N-C active sites in the carbon matrix results in excellent stability and tolerance toward any methanol fuel that may crossover to the cathode, while the Pt/C catalyst suffers from methanol poisoning and aggregation/leaching out during long-term operation.
This material also showed excellent performance as a catalyst in a zinc-air battery. In a zinc-air cell, the catalyst is a key component that reduces oxygen molecules in the air to oxide or hydroxide ions, which react with zinc. The low-cost Co-N-C carbon catalyst showed electrochemical performance similar to the expensive platinum-based catalysts used in metal air batteries.