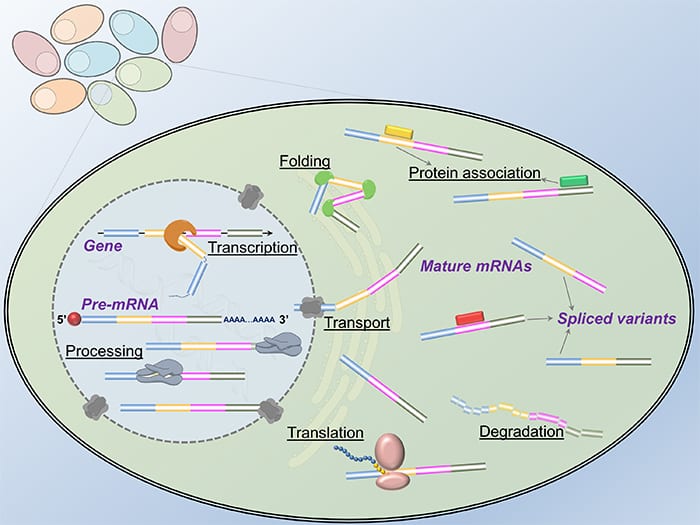
No two leaves are alike, and no two cells in our body are alike even if their genomes share exactly the same sequence information. Seeking a comprehensive understanding of the mechanisms accounting for the cellular heterogeneity is tremendously appealing to the scientific community. For a single cell to carry out its full function, a specific set of genes has to be activated to generate proper protein products. During this process, the transition of genetic codes from DNA to protein is mediated by RNA. The existence of RNA in the “Central Dogma” provides a necessary mechanism for a cell to diversify its protein products as the same DNA sequence of a gene can give rise to different RNA transcripts by selectively discarding or keeping specific regions, called exons, a phenomenon called alternative splicing. However, considering the limited spatiotemporal resolution of the conventional methods in molecular biology, it is extremely challenging to capture how the RNA molecules are transcribed and processed inside single intact cells.
Modern optical microscopy and nanotechnology enable single-cell quantification and characterization of RNA molecules since it greatly improves the detection accuracy and confidence. In situ analysis of transcriptome and pre-mRNA alternative splicing at the nanoscale therefore becomes a powerful tool to inspect gene expression, map regulatory networks, and classify cell types. The continued progress in RNA research via nanoscopic methodologies not only facilitates mechanistic studies of life science but also advances medical theranostics.
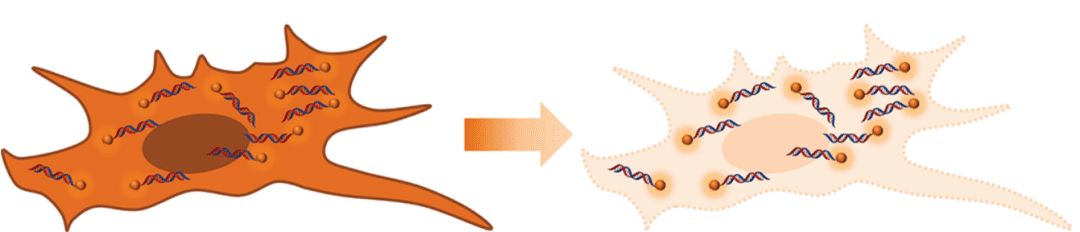
The scheme of improving signal-to-background ratio in single cells.
In the review article “Beyond quantification: in situ analysis of transcriptome and pre-mRNA alternative splicing at the nanoscale” in WIREs Nanomedicine and Nanobiotechnology, the authors discuss the most recent developments in single-cell RNA analysis through optical microscopy and nanotechnology. The innovative platforms combining state-of-the-art microscopy methods with various contrast-generating nanomaterials are highlighted. In parallel, a comparison between in situ analysis and single-cell sequencing, as well as their complementary functions are briefly discussed. Owing to the advances in instrumentation, opportunities abound in enhancing our knowledge of physiology and pathology relating to RNA biology at an unprecedented resolution.