Cardiovascular diseases are, as we speak, the major cause of death in the world and atherosclerosis is the most prominent among the diverse group of cardiovascular diseases. This chronic inflammatory disease process evolves for decades before first symptoms are noticed and causes myocardial infarction or stroke in the worst case. It is believed that progression of atherosclerosis is promoted by high lipid levels in the blood stream, which trigger the lipid uptake and formation of lipid droplets in macrophages – a type of white blood cell – of the arterial wall. The process finally results in foam cell formation when lipid droplets fill the cytoplasm, a stage at which the increased lipid concentrations can become cytotoxic.
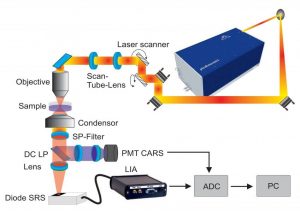
Scheme of the setup used for CRS imaging.
To overcome limitations of other study methods like fixative staining methods, label-free imaging techniques based on Raman scattering have been employed to visualize lipid droplets in living cells. The Raman effect is an inelastic scattering process exciting molecular vibrations which makes Raman spectroscopy is a very powerful method to determine subtle compositional changes in biological samples. Since lipids exhibit a large Raman scattering cross-section, the method is specifically suited for visualizing the distribution and composition of these lipids.
A team of German scientists studied the dynamic process of the storage of fatty acid – a category of lipids – in human macrophages with real-time data acquisition by following living cells with Raman spectroscopic imaging: they used both fast spontaneous Raman and Stimulated Raman Scattering (SRS) imaging . The team tracked individual cells over several hours to investigate uptake dynamics and storage efficiency at the single cell level and for entire cell populations. By combining results from spontaneous Raman and SRS imaging the team was able to present a complete overview of the incubation and uptake behaviors of living macrophages. By employing both techniques, they were able to show, for the first time, that the uptake kinetics of fatty acid by macrophages differ between individual cells, which brings us a step closer in understanding the workings of cardiovascular diseases.

















