Chemotherapy is an important treatment modality for cancer, however, chemotherapeutic drugs are associated with severe side effects, while their therapeutic efficacy tends to be suboptimal. Targeted drug delivery plays an important role in improving the efficacy of chemotherapy by enhancing tumor disposition of cytostatic drugs while decreasing the accumulation in healthy organs. Polymeric micelles are among the most successful drug delivery systems for targeted delivery. Amphiphilic block copolymers self-assemble in aqueous solution into micelles consisting of a lipophilic core and hydrophilic corona. The hydrophilic corona ensures their physicochemical stability while the lipophilic core can be loaded with hydrophobic chemotherapeutic drugs such as paclitaxel and docetaxel. This makes polymeric micelles highly suitable delivery systems for chemotherapeutic drugs and currently there are more than ten nanoformulations based on polymeric micelles in clinical trials for the treatment of different types of cancers.
Nevertheless, research performed by various groups has pointed out that, although polymeric micelles have shown their pharmaceutical potential, several limitations hamper their clinical translation. The most serious drawbacks include the poor micelle stability and insufficient drug retention in the circulation and biological fluids, which overall result in rapid blood clearance of drug-loaded polymeric micelles and premature drug release, and consequently poor tumor targeting efficiency.
 A recently published feature article authored by researchers from South China University of Technology (China), Utrecht University (The Netherlands), RWTH Aachen University (Germany) and University of Twente (The Netherlands) comprehensively discusses the reasons for the poor targeting efficiency via polymeric micelles and moreover the approaches that have been explored to tackle this problem.
A recently published feature article authored by researchers from South China University of Technology (China), Utrecht University (The Netherlands), RWTH Aachen University (Germany) and University of Twente (The Netherlands) comprehensively discusses the reasons for the poor targeting efficiency via polymeric micelles and moreover the approaches that have been explored to tackle this problem.
The dilution of polymeric micelles after intravenous injections and/or interactions between polymer building blocks and blood components lead to disintegration of polymeric micelles. Also, translocation of loaded drugs from the micellar core to biological components (e.g, plasma proteins) during circulation results in premature drug release. These two reasons contribute to short blood circulation of drug-loaded polymeric micelles and therefore low drug targeting efficiency.
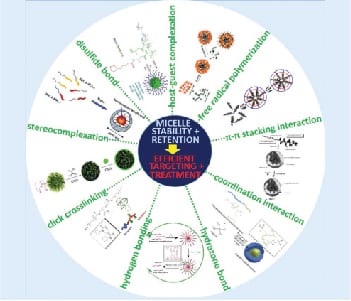
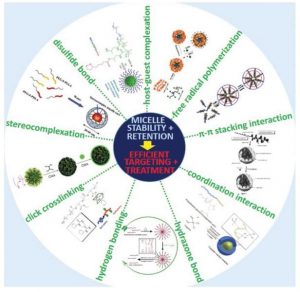
As reviewed in this article, several physical (e.g. π-π stacking, stereocomplexation, hydrogen bonding, host-guest complexation and coordination interaction) and chemical (e.g. free radical polymerization, click chemistry, disulfide and hydrazone bonding) strategies have been applied to improve micelle stability and drug retention. And these efforts have ultimately resulted in better polymeric micelles for drug delivery suitable for clinical translation.