Next generation polymer-based applications ranging from flexible microelectronics to drug delivery systems require specific and tunable material properties. Desired material properties are obtained by controlling the molecular weight characteristics, molecular architecture and composition of the polymers themselves. For example, block copolymers which contain two chemically distinct polymers gives rise to a final material with a combination of properties originating from both independent blocks. Unlike a simple blend of polymers, block copolymers are covalently bound and therefore do not macro phase separate. In fact, block copolymers do phase separate – but only on the nanoscale. This thermodynamically predictable self-assembly gives rise to specialized applications such as nano-porous membranes, polymer templates for nano metallic rods and high surface catalytic supports, to name but a few examples.
Nitroxide mediated polymerization (NMP) is an industrially friendly technique which often only requires a simple unimolecular initiator to obtain polymers with low molecular weight distributions and high chain end livingness: factors which are necessary to obtain well defined block copolymers. NMP is versatile; it can be performed in aqueous systems, results in polymers that are biologically benign, is used to grow polymers off of inorganic surfaces, and can synthesize polymers for electronic applications without any specialized purification required. Over the years, researchers have been finding ways to use NMP to polymerize a variety of functional monomers including styrenics, acrylates, acrylamides and even methacrylates.
The study by B. Lessard and co-workers at the University of Ottawa shows the first example of controlled synthesis of poly(N-(isobutoxymethyl) acrylamide) (poly(IBMA)) homopolymers and block copolymers by controlled polymerization techniques. N-(Isobutoxymethyl) acrylamide is a water-insoluble, commercially available monomer. In this study, the authors first explored the controlled synthesis of poly(IBMA) homopolymers with narrow molecular weight distribution and linear increase in number average molecular weight (Mn) versus monomer conversion. The authors developed a linear correlation between the polymerization temperature and the polymerization rate. Secondly, the authors used these poly(IBMA) homopolymers as macroinitiators for the synthesis of styrene block copolymers, demonstrating that even at high conversion the poly(IBMA) homopolymers are pseudo-living and can be used to engineer well defined block copolymers with a low concentration of unreacted chains.
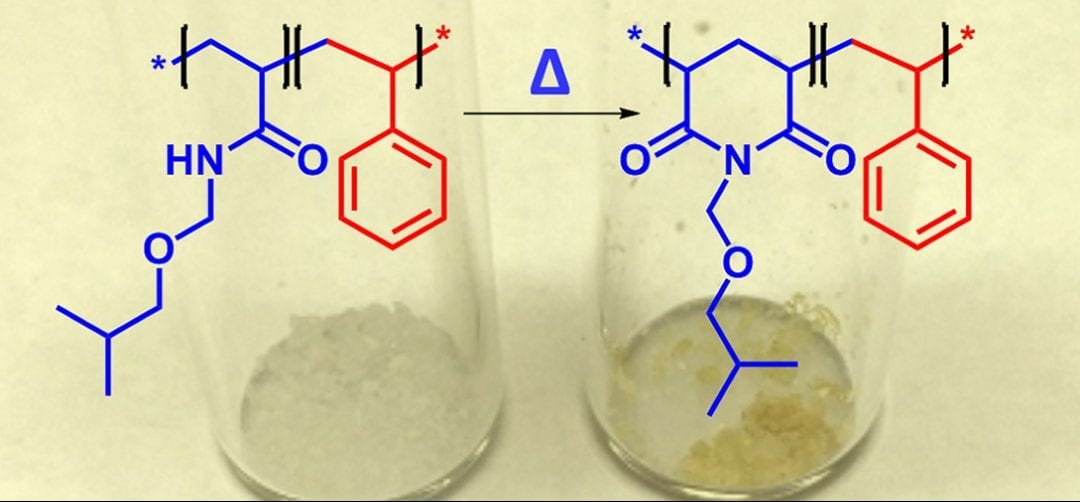
Finally, Lessard and coworkers the authors sought out to characterize some fundamental thermal properties of the poly(IBMA) homopolymers and block copolymers, which have not yet been reported in the literature. They determined the glass transition temperature (Tg) of these polymers to be very similar to that of other commercial polymers, such as poly(styrene) and poly(methyl methacrylate). When performing thermogravimetric analysis (TGA) the authors observed that two different degradation events take place. When studying the resulting gas exhaust from the decomposition by Fourier Transform Infrared Spectroscopy (FTIR) it could be identified that the poly(IBMA) homopolymers and block copolymers underwent an imidization reaction at >200 °C followed by a full backbone decomposition >350 °C.
It is interesting to note that previously poly(acrylamide), which contains primary amines, were the only acrylamides that were known to undergo these types of imidization degradation reactions while poly(N-isopropylacrylamide), which contains secondary amines, and poly(N,N-dimethylacrylamide) which contains tertiary amines, do not. This study suggests that polymers, other than poly(acrylamide), such as poly(IBMA) can undergo imidization degradation mechanisms.