With structural and biological advantages over previous models, a haystack-like nanostructure implanted with neural stem cells prompted repair of a spinal cord injury in mice.
In basic and clinical research, therapeutic efforts after a life-altering spinal cord injury have usually targeted some form of self-healing induced through external agents, be they chemical or biological. Stem cell therapy, which stimulates neural stem cells to drive nerve regeneration, has garnered a lot of attention in the past. But its benefits in practice remain to be seen.
“There are many challenges in SCI [spinal cord injury] therapy, such as how to achieve efficient nerve regeneration, how to achieve specific treatment, and whether the transplantation of cells or materials will bring new problems, and so on,” said Yuanhua Sang, a researcher at Shandong University, in China, an author on the study published in Advanced Functional Materials.
The transplantation of neural stem cells, while a strong contender for treating spinal cord injury, can be plagued with problems. In order to help mend the spinal cord, these neural stem cells must thrive and be transformed into other fully developed neural cells. But when transplanted, neural stem cells can have a hard time surviving and differentiating into other types of neural cells that are needed for the regeneration of the damaged spinal cord.
Channeling nutrients through nanobelts
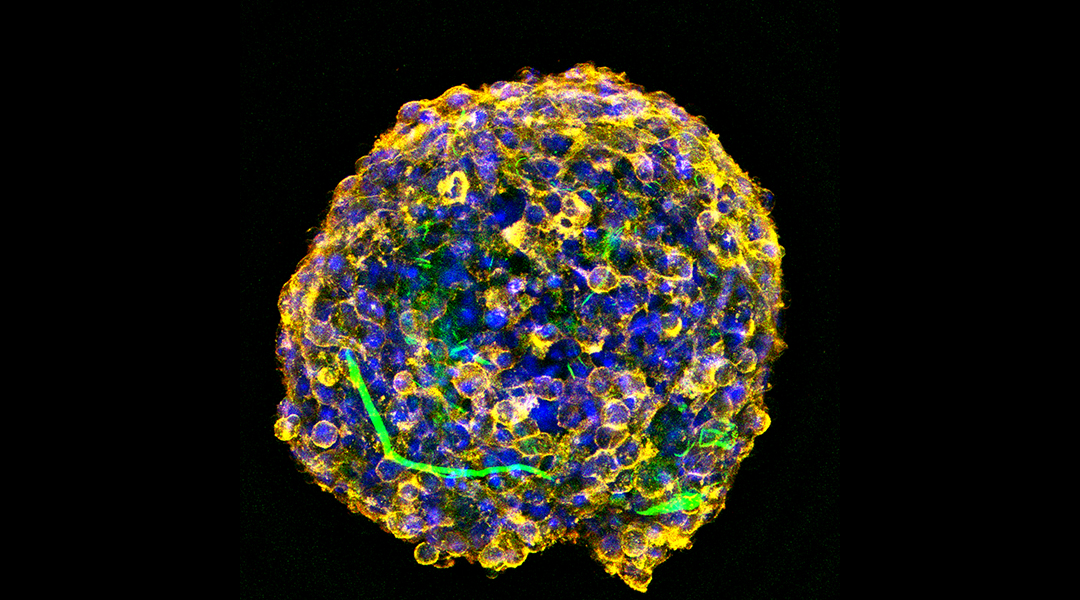
One method of neural stem cell transplantation that is being explored to treat spinal cord injury uses cell spheroids — a three-dimensional cluster of cells, usually with some type of nanomaterial for structural support and protection.
“In the prevailing method of assembling stem cells into a 3D spheroid, oxygen, nutrients, etc., cannot be successfully transported into the core of the spheroid,” said first author Min Hao, a researcher at Shandong University, in China. When the implanted cells are unable to access oxygen and nutrients, cell death takes place, making any repair efforts moot.
In order to combat this issue, Sang, Hao, and their colleagues designed and developed a new type of cell spheroid. They used hydroxypatite, a calcium phosphate mineral commonly found in bone and commerically used to repair it, to make straw-like nanostructures. Due to their resemblance to belts, these nanostructures are termed nanobelts.
Next, these nanobelts were coated with polydopamine, a sticky, polymer coating akin to the secretions of mussels, and superparamagnetic iron oxide, a type of magnetic nanoparticle that are magnetic when placed in an external magnetic field. When combined with mouse neural stem cells, the haystack-like hydroxypatite nanobelts became embedded with cells. While the polydopamine helped the cells adhere better to the nanostructure, the iron oxide kept the spheroid magnetically targeted to specific regions during transplantation.
Through a series of in vitro and in vivo experiments, the researchers found that the spheroids were structurally sound, biocompatible, and stable. Moreover, these nanostructures played the role of a “nutrient transport belt,” said Hao.
Averting cell death
By ferrying nutrients, oxygen and other soluble molecules, the haystack nanobelt’s structure averted cell death common in earlier models. In parallel, the biological function of the nanobelt helped the transplanted neural cells survive, differentiate, mature and spread, rapidly.
“The dual-functional HAp [hydroxypatite] nanobelts also promote cell differentiation, which was integrated in the 3D cell spheroids,” added Hao. Moreover, mouse neural stem cells transplanted in the form of 3D cell spheroids were able to foster repair after a spinal cord contusion injury. The researchers suggest that these modified 3D cell spheroids could offer a therapeutic approach for spinal cord injuries caused for instance, by a car accident.
“The strategy of 3D multifunctional HAp nanobelt haystack assembled hybrid spheroids will bring a new prospect to therapeutics beyond SCI repair,” said Hao. “It would promote stem cell therapeutics, not only in the SCI but also in other injurious diseases.”
Such cell spheroids could hold promise in repairing bones as well as be applied in other cellular settings, suggested Hao.
“The multifunctional regulation of the cell spheroid, including the neural microenvironment after transplantation in vivo, is something we need to improve, and the mechanism in vivo should be further studied,” added Sang. “Now, we are working with researchers in the medicine and biology fields to improve it.”
Reference: Min Hao, et al., Multifunctional Hydroxyapatite Nanobelt Haystacks Integrated Neural Stem Cell Spheroid for Rapid Spinal Cord Injury Repair, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202214869