The cornea is the outermost part of the eye composed of three distinct layers. The middle, stromal layer, constitutes up to 90% of the corneal thickness. Optimal functionality of the stroma is important for the transparency and consequently the vision. The function of the stroma is mainly dependent on: 1) the well-ordered topographical arrangement of extracellular matrix (ECM), and 2) the mechanical strain created by the corneal shape and the intraocular pressure.
Keratocytes are the corneal fibroblasts residing in the stroma. During in vitro traditional 2D cell culture conditions, keratocytes easily differentiate and lose their typical features. In order to overcome the limitations of 2D monolayer cell cultures, Danielson and colleagues from Umeå University, Sweden, report in their recent article in Advanced Healthcare Materials, the development of a biomimetic 3D corneal in vitro model that combines the topographical and mechanical cues similar to those of the native cornea.
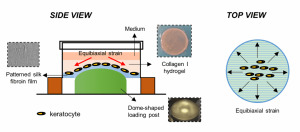
Keratocytes are seeded on silk fibroin-patterned constructs with a collagen I hydrogel on top (side view). The whole construct is then exposed to equibiaxial strain (top view).
The biomimetic 3D corneal model consists of keratocytes cultured on a silk fibroin-patterned construct to achieve the topographical arrangement. Collagen I hydrogel is placed on top to get the 3D environment. This construct was exposed to 3% dome-shaped equibiaxial strain. The whole construct, including the strain, enhanced the expression of keratocyte and ECM markers, and better maintained the keratocyte phenotype as compared to the traditional 2D culture conditions. It not only provides an in vitro 3D culture model that improves the current 2D culture conditions for corneal studies, but also offers valuable insight for the future development of biomimetic tissue-engineered corneal replacement grafts for in vivo applications.