Scientists have developed a new 3D cell culture device made from soft, porous microgel particles that can mimic the effects of pressure on stem cells. This platform allows researchers to study and even influence how stem cells behave under different conditions, potentially leading to advances in regenerative medicine, tissue engineering, and disease research.
The platform, dubbed 3D-PRESS, has the potential to facilitate the production of large numbers of stem cells or preconditioned cells, which could ultimately enhance the efficiency and effectiveness of different cell therapies, treatments that involve introducing new cells to patients to combat injury or disease.
Stem cells have potential in regenerative medicine thanks to two key properties. Firstly, they are self-renewing, meaning they are capable of creating more cells like themselves. Secondly, stem cells are capable of becoming a multitude of other cell types through a process called “differentiation.” They are the only cells in the body with the natural ability to do this.
Already found in most tissues of the body, stem cells are essential for the maintenance of tissue as well as for repair after injury. In regenerative medicine, stem cells can be introduced and guided to become specific cells and thus used to regenerate and repair tissues that have been damaged or otherwise impacted by disease.
“One of the most significant uses of stem cells with micro-scale programmable materials is in regenerative medicine, where they can support the repair and regeneration of damaged tissues,” said Berna Özkale Edelmann, assistant professor at the Technical University of Munich. “For example, they are being used to grow stem cells into cartilage or bone to treat injuries or degenerative diseases like osteoarthritis.”
The difficulty with stem cells
One of the difficulties with stem cells is introducing them to the patient’s body without triggering an immune response, which can drastically reduce their regenerative capability.
“Micro-scale, programmable materials have become essential tools for stem cell transplantation as cell delivery vehicles as they protect cells from harsh conditions like immune attacks and nutrient shortages,” said Nergishan İyisan, who is the PhD student working on the project. “Acting as a protective shell, they help stem cells survive, stay in place, and integrate with surrounding tissues. Additionally, their small size allows them to be injected directly into the body using minimally invasive methods.”
Similarly, Özkale added, micro-scaffolds containing stem cells or their secreted factors can be used to control immune response. These micro-scale scaffolds can be created using porous microgels capable of protecting stem cells as they are introduced to a patient’s body and “sneaking” them past the patient’s immune response. Acting as tiny, programmable carriers for stem cells this enables the 3D culturing of cells that are highly biocompatible with the body. They are considered programmable because their properties can be tailored by modifying the biomaterials they are made from.
“For example, biomolecules can be added to enhance cell adhesion or growth factors can be incorporated to promote cell growth and differentiation,” İyisan said. “This versatility makes microgels an essential tool in regenerative medicine and stem cell transplantation.”
Small structures. Big impact
There are still many challenges associated with controlling stem cell behavior in 3D environments. One key issue is the natural variability within stem cell populations, as individual cells often behave differently, making it difficult to achieve consistent outcomes.
“While we have a solid understanding of the biochemical signals that influence stem cells, the effects of mechanical signals remain less explored,” Özkale said. “Delivering controlled mechanical cues to individual cells, particularly in a 3D environment, is challenging.”
The team designed 3D-PRESS to provide just such a mechanical stimulation of cells in microgels.
The 3D-printed pressure chamber applies precise and adjustable pressure to individual stem cells encapsulated within microgels. It consists of a chamber capable of holding thousands of microgels and a lid connected to a pneumatic actuation system. Furthermore, 3D-PRESS is made from a transparent, biocompatible material that allows researchers to make real-time observations of how stem cells respond to mechanical forces.
“Using the 3D-PRESS, we can study how mechanical forces affect stem cells in a 3D environment, namely microgels, providing valuable insights into their behavior and mechanotransduction processes,” İyisan said. “It allows us to apply precise pressure to a large number of cells simultaneously while maintaining their 3D microenvironment.
“This technology will be crucial for programming and differentiating stem cells in a high throughput manner under robust programming.”
The team tested the platform using compressed air up to 400 kilopascal (kPa). For context, 1 kilopascal corresponds to about 1% of atmospheric pressure at sea level. An integrated pressure sensor allowed the team to take measurements that validated the device’s robustness and durability, demonstrating that 3D-PRESS was able to withstand multiple cycles of use over hours and days.
“We directly implemented the platform to study mechanotransduction, ensuring its effectiveness in delivering controlled mechanical stimulation to stem cells,” İyisan said. “There was no time lag in delivering pressure to the cells, allowing us to perform mechanical stimulation continuously over hours.
“Biocompatibility tests confirmed that the cells remained viable for up to a week. For mechanotransduction, the platform enables us to investigate both short- and long-term effects, meaning we could observe immediate changes caused by mechanotransduction as well as prolonged effects over time.”
Pushing PRESS’ limits
The team is currently expanding 3D-PRESS to work with human pluripotent stem cells, a particularly potent type of stem cell that generally only exists during early embryonic development. This will increase the variety of cell types that can be generated using this technology.
“This will help medical practitioners by providing preconditioned or specialized cells in large quantities for use in therapies,” Özkale explained. “For patients, this advancement could support the development of injectable stem cell-based therapeutics, offering more efficient and personalized treatment options.”
The researchers anticipate that it will take at least five years to realize these applications. During this time, they intend to scale up the platform so it can encompass many more stem cells, something essential to applying it in cell therapy. The team is also working closely with collaborators, including clinicians, to help develop and prepare therapies that will employ the platform.
“To enable wide-scale use, the 3D-PRESS needs to be tested with multiple cell types to validate its versatility. While the platform is robust in its current form and suitable for millions of cells, it must be scaled up to handle billions of cells required for transplantation applications,” Özkale said. “This involves increasing the size of the chip and ensuring the system can operate in parallel without losing its precision or efficiency.
“Scaling up the process to produce large numbers of pre-differentiated cells or to ‘train’ many stem cells simultaneously is a significant limitation, as therapeutic applications require a large number of cells.
Reference: B. Özkale, et al., Mechanoactivation of Single Stem Cells in Microgels Using a 3D-Printed Stimulation Device, Small Methods., (2024), DOI: 10.1002/smtd.202400272
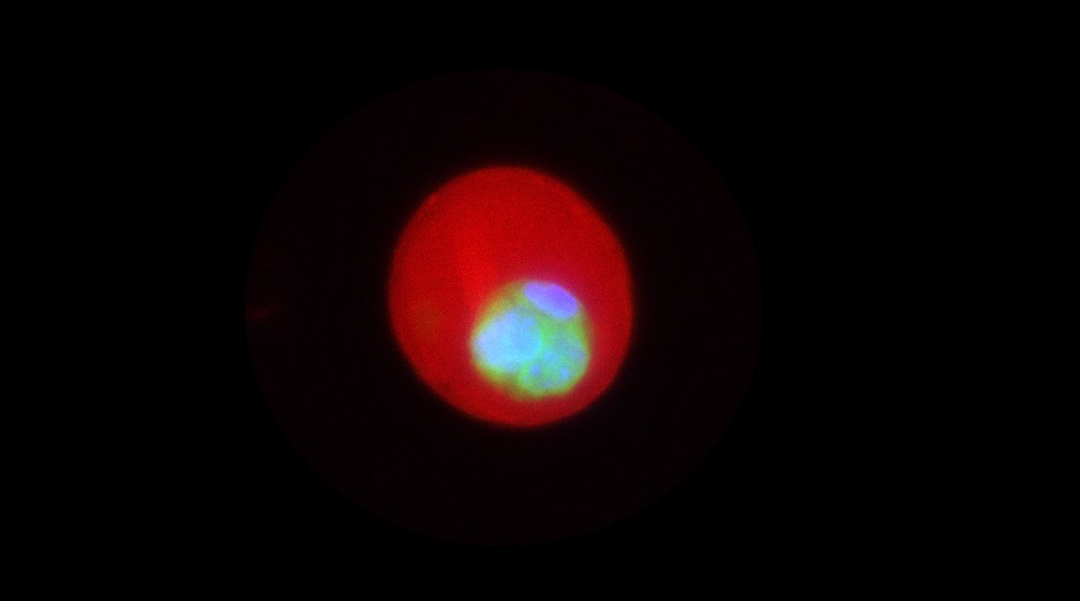
Feature image: Encapsulated mesenchymal stem cells (MSCs) within alginate microgels. The red fluorescence marks the alginate microgels, which provide a 3D culture environment for MSCs. Green represents the cytoplasm of the cells, while blue indicates the nuclei. The image captures a single MSC proliferating into a multicellular cluster one week post-encapsulation.