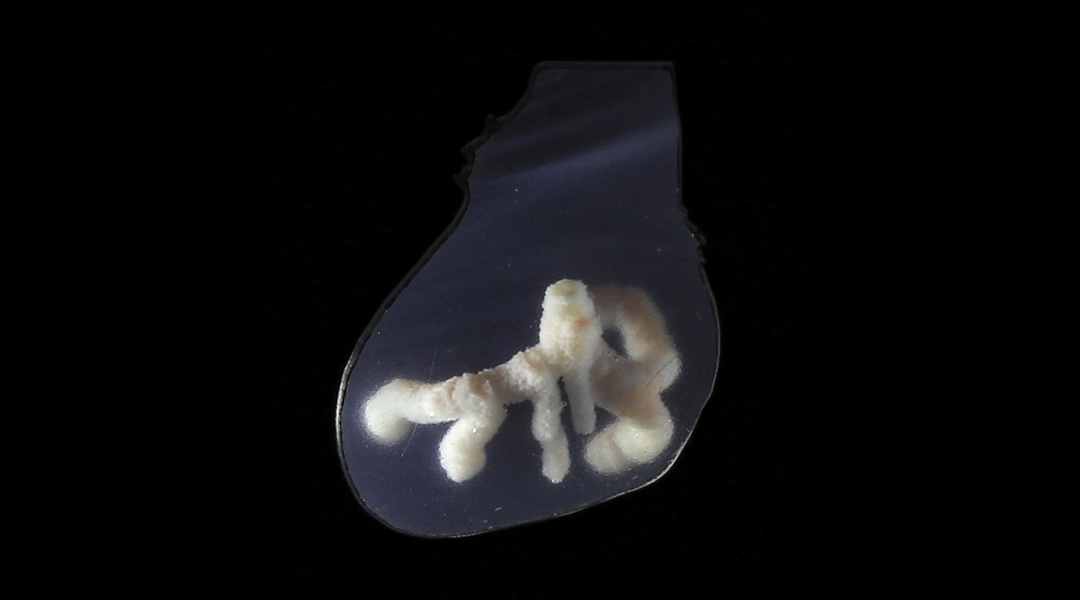
Researchers have developed a technique to 3D print blood vessel networks that emulate the body’s natural network of arteries, veins, and capillaries crucial for transporting blood and nutrients. This method could make lab-manufactured organ transplants not just a possibility but a viable reality, potentially transforming how we approach organ donation and transplantation in the future.
Blood vessels are made up of concentric cell layers. The innermost layer, the intima, is a barrier formed by endothelial cells, supported by smooth muscle cells that offer strength. These vessels branch hierarchically — a structure that’s been challenging to replicate artificially.
Various iterations of 3D printing for human tissues and organs have been developed over the last two decades. However, printing organs with viable, vascular networks has remained an elusive task. While 3D printing of vascular networks was first explored in 2011, the resulting constructs were made without the use of cells.
Despite improvements, printed vessels have not come close to mimicking natural blood vessels. As a result, it has been daunting to craft printed tissues and organs that stay viable over the time needed to model disease or test therapies.
What goes into 3D printing heart tissue
Jessica Lewis, a researcher at the Wyss Institute and the Paulson School for Engineering and Applied Sciences at Harvard University, and her team have worked on printing vascularized human tissues for more than a decade.
In 2014, the team first published evidence of vascular channels that allowed the flow of liquids. They further advanced this method, now called sacrificial writing into functional tissues (SWIFT), to 3D print blood channels within tissues with cell densities that matched those found in the human body. In this method, a sacrificial gelatin ink is printed into tissue at low temperatures.
When the functional tissue is warmed up, the gelatin liquefies, leaving behind channels that are open to the flow of fluids. But these channels lacked the layers of smooth muscle and endothelial cells that make up the walls of native blood vessels.
With the latest step forward in their technique, the team has finally 3D printed blood vessels that have multiple cell layers just like natural blood vessels. “We introduce an improved method, known as coaxial sacrificial writing into functional tissue (co-SWIFT), which marries coaxial 3D printing and SWIFT to enable, for the first time, 3D printing of networks of multi-layer vasculature embedded within both cell-dense or acellular materials,” Lewis wrote in an email.
The team developed a specialized coaxial nozzle that is capable of depositing two different inks, layered within each other, into a support matrix. The two layers can be controlled independent of each other, to lay down one or more stiff, collagen-based outer shells and a gelatin-based inner core layer.
Since the inner channel protrudes out of the outer channel, it is able to pierce vessels that were printed before, creating interlinked vascular networks. Originally printed at low temperatures, the matrix is heated to 37℃. The molecules in the matrix and outer shell crosslink, while the gelatin melts away, creating empty channels.
The researchers tested the ability of co-SWIFT to 3D print blood vessels in different materials. To optimize their technique and easily visualize the networks, they initially used a transparent hydrogel matrix.
In a separate experiment, they also printed the artificial blood vessels in a porous collagen-based matrix, which was meant to imitate fibrous muscle tissue. After testing their collagen- and gelatin-based inks, the researchers evaluated cell-based inks.
They printed the vessels using a shell ink with smooth muscle cells. Once the inner gelatin layer was melted away, endothelial cells were passed through the empty channels to replicate the inner layer of native blood vessels. After seven days, the endothelial layer formed a strong barrier.
How did the artificial blood vessels do?
After this success, they researchers tested the printing and performance of the artificial blood vessels within living cardiac tissue. For this experiment, they 3D printed a network of blood vessels within cardiac organ building blocks. These organ building blocks are dense clusters of functional, human heart cells. The resulting blood vessels had a dual layer of cells — endothelial and smooth muscle — just like in human blood vessels.
After setting up the flow of a blood-like liquid within the cardiac tissue, it began contracting synchronously, mimicking a beating heart. On passing cardiac drugs through these vessels, the tissue responded appropriately. For instance, isoproterenol, a drug used to treat bradycardia wherein the heart beats slowly, made the cardiac tissue beat twice as fast.
On the other hand, using blebbistatin, a drug known to limit the contractile activity of heart cells, ceased the beating of the cardiac tissue. Furthermore, the researchers printed a replica of an actual person’s left coronary artery, extracted from a patient’s imaging results, into cardiac organ building blocks. “This highlights co-SWIFT’s ability to deliver patient specific solutions,” added Lewis.
“co-SWIFT unlocks our ability to generate biologically relevant blood vessels within functional human tissues,” co-author Paul Stankey, a researcher at Wyss, wrote in an email. “This is a vital step towards our goal of translating engineered tissues to the clinic and, ultimately, building whole organs for therapeutic use.” These biomimetic vascular networks could help print human tissues and organs that can be used to model disease, test drugs, and develop therapies.
While co-SWIFT can print large blood vessels that keep cardiac tissue alive, the researchers hope to eventually print smaller vessels that resemble capillaries, which are key to proper organ function. They also intend to test tissues with 3D printed vessels in animals.
“On the scientific side, functional maturation of the organ is the main hurdle remaining now that we can build human tissues at a therapeutically relevant size using co-SWIFT,” added Stankey. “On the industrial side, finding and implementing a cell source that will overcome immune rejection at an affordable cost will be the key to bringing these lab-manufactured tissues to patients at scale.”
Reference: Paul P. Stankey, et al., Embedding Biomimetic Vascular Networks via Coaxial Sacrificial Writing into Functional Tissue, Advanced Materials (2024). DOI: 10.1002/adma.202401528