Image credit: Ameloot Group
The current COVID-19 pandemic has made the need for rapid diagnostic tests abundantly clear. Rapid self-tests are being used throughout the world, and are based on a lateral flow test set-up. Samples taken from the throat or nasal cavity are dissolved in a solvent, and applied to the test kit. Absorbent material in the kit moves the sample downstream and brings it in contact with an antibody. If the target antigen is present, the antibody reacts and a colored line appears. Pregnancy tests work in a similar way.
The advantage of these tests is that they are cheap and do not require any specialized equipment or technicians. However, beyond situations that require a simple “yes or no” answer, these lateral flow devices have limited application.
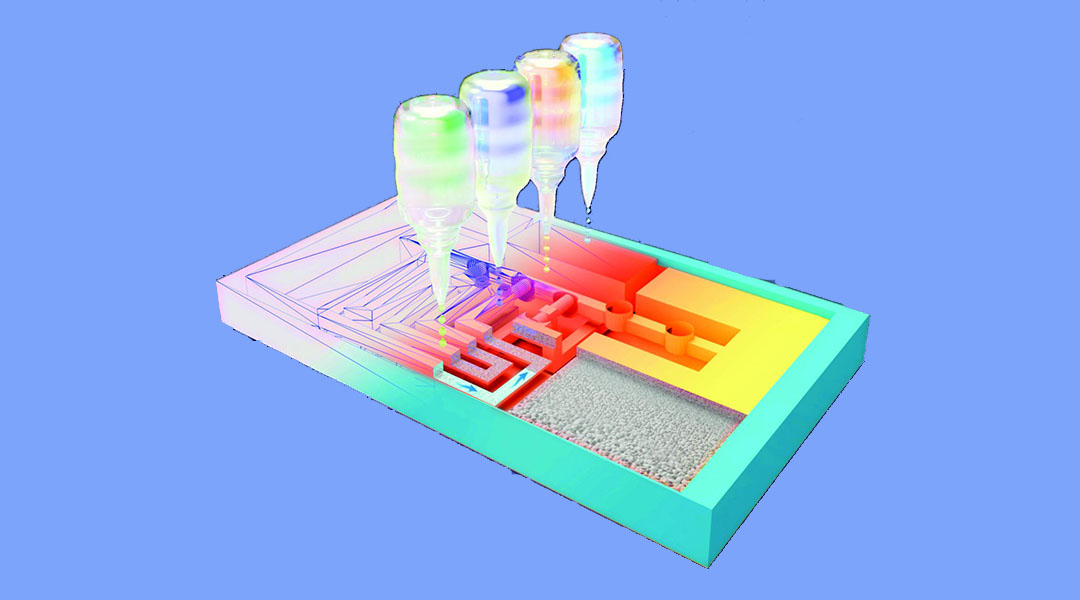
Researchers at KU Leuven in Belgium, led by Professor Rob Ameloot, have developed a new 3D printing technique that extends the possibilities of lateral flow testing by creating a new type of platform with more capabilities.
To do this, they fabricated a 3D version of a lateral flow test based on a small block made from a porous polymer. Inks with different properties were then printed at precise locations. In this way, a network of channels and small locks was printed that let the flow through or blocked it where and when necessary, without the need for moving parts. During the test, the sample is automatically guided through the different test steps. This structure allows even complex protocols to be followed.
“The great thing about 3D printing is that you can quickly adapt a test’s design to accommodate another protocol, for example, to detect a cancer biomarker,” said Cesar Parra-Cabrera, one of the authors on the study published in Advanced Materials. “For the 3D printer it does not matter how complex the network of channels is.”
To evaluate the utility of their technique, the team applied it to the reproduction of an ELISA test (Enzyme-Linked Immunosorbent Assay), common in biology and medicine, and used it to detect immunoglobulin E (IgE), a biomolecule usually measured to diagnose allergies. In the lab, ELISA tests requires a skilled technician to run, involving several steps with different rinses and a change in acidity to complete.
However, in the current study the team was able to run this entire protocol using a printed test kit the size of a credit card, which is also affordable and easily scalable. “In our lab, producing the Ig E prototype test costs about $1.50 [US], but if we can scale it up, it would be less than $1 [US],” said Parra. The technique not only offers opportunities for cheaper and faster diagnosis in developed countries, but also in countries where the medical infrastructure is less accessible and where there is a strong need for affordable diagnostic tests.
The research group is currently designing its own 3D printer, which will be more flexible than the commercial model used in the current study.
“An optimized printer is kind of like a mobile mini factory which can quickly produce diagnostics. You could then create different types of tests by simply loading a different design file and ink. We want to continue our research on diagnostic challenges and applications with the help of partners,” concluded innovation manager, Bart van Duffel.
Reference: Clement Achille, et al., 3D Printing of Monolithic Capillarity‐Driven Microfluidic Devices for Diagnostics, Advanced Materials (2021). DOI: 10.1002/adma.202008712