The peripheral nervous system is like a network of electrical wires. Made up of nerves, it connects the brain and spinal cord, which constitute the central nervous system, to the rest of the body.
The peripheral nervous system controls sensations such as touch, heat, and pain, and controls the movements of muscles. When peripheral nerve injuries occur, their connection with the central nervous system is lost, which can lead to severe impairment of motor and sensory functions.
Peripheral nerve injuries have long posed a challenge for effective regeneration. The most common therapy for this kind of injury is a surgical procedure called neurorrhaphy that reconnects both ends of the damaged nerve. But when “long-gap injuries” occur and a significant part of the nerves are lost, this surgery cannot be performed.
The only solution is to perform an autologous nerve grafting in which a section of healthy nerve is taken from another part of the patient’s body and used to bridge or repair a damaged nerve. But this treatment comes with limitations, such as complications that may arise from the area where the nerve is extracted and the limited availability of donor nerves.
A biodegradable nerve scaffold
Scientists around the world are studying innovative techniques that involve artificial scaffolds, structures made of biocompatible materials designed to support the regeneration and growth of the nerves, acting as a temporary support system that provides a framework for cells to adhere to and grow upon.
In a recent study published in Advanced Healthcare Materials, scientists have introduced a fully biodegradable nerve scaffold with the ability to eliminate any material residues, thereby negating the necessity for subsequent retrieval procedures. This is a significant advancement in simplifying the treatment process for nerve injuries.
“Our work proposes a fully bioresorbable and conductive nerve scaffold, which offers both structural guidance and electrical cues to promote nerve regeneration,” said Lan Yin, associate professor at the School of Materials Science and Engineering at Tsinghua University, Beijing, China, and lead author of the study. “This scaffold could be utilized to facilitate the regeneration of peripheral nerves in cases of long gap injuries.”
Silicon provides a boost
For it to be effective, a nerve scaffold should contain materials that help guide damaged nerves from one end to the other, help with the adherence and growth of Schwann cells which wrap around the nerves in a sheath-like structure called the myelin sheath, and provide a solid structure where the nerves can regrow.
To build a nerve scaffold that meets all these characteristics, Yin and his team used four layers of different materials, each of which provides a specific and necessary function.
As the most internal layer, they used fibers that serve as structural guidance to assist nerve growth in the desirable direction. Then they added N-type silicon membranes designed to conduct electricity, the purpose of which is to enhance endogenous electrical cues and facilitate nerve regrowth. For the third layer, the team chose a flexible material that serves as the substrate of the entire framework and provides inner mechanical support. And as the outer layer, the scientists used a porous structure that ensures the penetration of nutrients to assist nerve repair.
With experiments using cultured mouse peripheral nerve cells, the team also showed that having N-type silicon material around can boost special factors that help nerves grow, make Schwann cells grow faster, and improve the neurons’ calcium activity, which is a sign of better neuronal functioning. These experiments helped determine that the addition of a conductive material into the scaffold, could improve nerve regeneration.
“By integrating additional strategies like advanced structure, neurotrophic factors, and self-powered electric fields, it [the scaffold] holds great potential to maximize therapeutic benefits of regenerative medicine,” said Yin.
It degrades after implantation
To test how fast their scaffold could degrades under biological conditions, the team put the scaffold in phosphate-buffered saline solution at pH 7.4, simulating a similar environment to what’s found in the body. When heated to 55°C, the scaffold was almost completely degraded after two months.
“We used accelerated conditions at 55°C to demonstrate complete degradation of the entire conduit,” said Yin. “If deployed at body temperature, degradation is expected to be slower. It probably takes around a year to degrade in the body.”
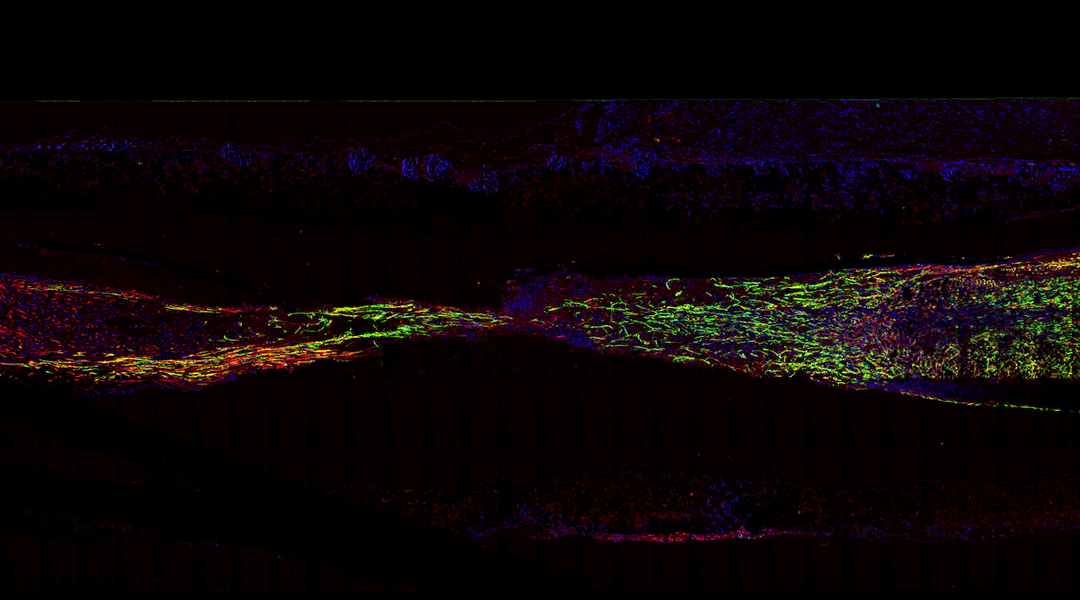
Using a mouse model of peripheral nerve injury, the scientists observed that by the third week after the scaffold implantation, the structure experienced partial degradation, primarily due to the breakdown of the outer porous layer. However, the inner flexible layer that serves as mechanical support remained almost unchanged even after 12 weeks, which ensures minimal mechanical deformation. The team explains that the slow degradation can help the full regeneration of the affected nerve before the structure completely resorbs in the body.
In the same mouse model, the scientists compare the performance of their scaffold with an autologous grafting, and with a scaffold without silicon membranes, then, without a conductive material.
First, they observed that the implantation of the scaffold did not produce significant inflammation, meaning the body tolerated its materials. Then, 12 weeks post-surgery, they observed that the newly formed myelinated nerve fibers in the mice with the team’s scaffold were evenly spread and had a higher concentration compared to those in the group without silicon membranes, and similar to the mice with autologous graft surgery.
Repaired muscle function
The team also tested if the regenerating nerve translated to real recovery of the affected muscle function.
For this, they measured different parameters of muscle function. At 12 weeks post-surgery they observed that the muscles of mice with the scaffold and autograft groups presented lower levels of atrophy than those of the control group without Si membranes.
When tracking the mice’s ability to walk, the scaffold group performed similarly to those with grafts, suggesting the biodegradable scaffold containing a conductive layer that accelerates nerve regeneration could be utilized as a replacement for autologous grafts.
“The work provides new routes toward the development of fully bioresorbable and electrically active materials to achieve desirable biological cues and microenvironments, offering important strategies of neural interface engineering for regenerative medicine,” said Yin.
This research marks a significant leap forward in the realm of regenerative medicine. The combination of bioresorbable materials and electrically active components in the nerve scaffold holds promise for achieving better neural interfaces and enhancing therapeutic outcomes for nerve injuries.
“We are working on experiments on larger animals to explore the potential for clinical translation,” said Yin.
Reference: Pengcheng Sun, et. al., A Bioresorbable and Conductive Scaffold Integrating Silicon Membranes for Peripheral Nerve Regeneration, Advanced Healthcare Materials (2023). DOI: 10.1002/adhm.202301859
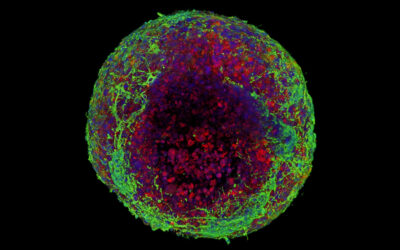
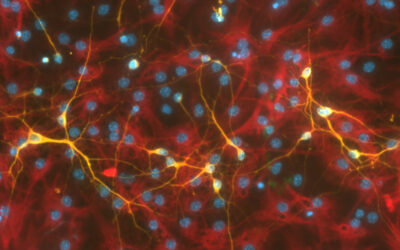
Feature image: Immunofluorescent image of the longitudinal sections of regenerated nerve tissues