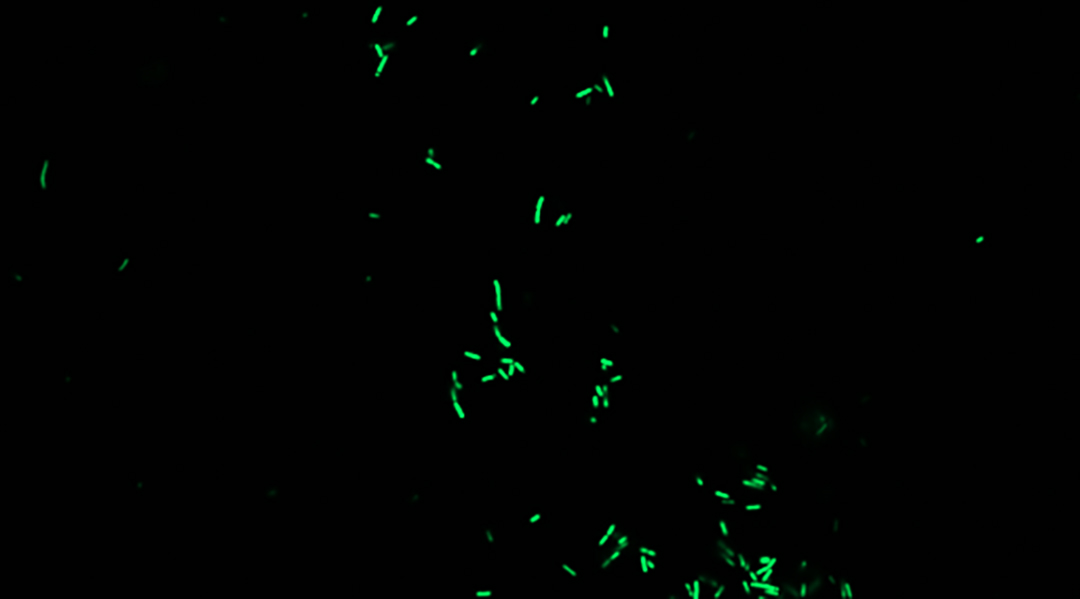
Scientists have built the first living biosensor to study the honeybee gut microbiome. Bacteria, like Escherichia coli (E.coli), can be programmed to serve as biosensors for gut microbiome research in mammals. However, this has never been done for honeybees.
To truly understand the complex interplay of factors that dictate the microbiomes’ role in the health of these threatened pollinators, the researcher team from the University of Lausanne in Switzerland needed to create a similar biosensor for their unique physiology.
The microbiomes’ vast reach
Reach of the microbiome extends beyond just digestion to behavior, development, and mental and physical health. In humans, researchers still struggle with the complexity of interactions that dictate how the hundreds of bacteria species living in our gut influence our lives.
In bees, however, this complexity is reduced. “Honeybees are a very nice model to study the microbiota,” said Audam Chhun, postdoctoral researcher at the University of Lausanne in Switzerland. According to Chhun, a honeybee taken from anywhere in the world will have the same eight to ten bacterial species in its gut.
Honeybees are also easy to work with in a lab and because they are social insects, their behaviors reveal links along the gut-brain axis. But, like all studies of the gut, getting a glimpse inside is no easy task.
Living biosensors
Advances in synthetic biology, which include our ability to manipulate and add genes into different organisms, first led to the ingenious idea of using gut bacteria as living sensors to report on the molecules and conditions of the gut.
These bacteria are engineered to produce proteins that act like simple circuits. In general, these circuits contain a sensing protein, which binds a target of interest and another signalling protein, which researchers can then visualize in the lab. These biosensors are innocuous and allow researchers to track the molecules present in the gut.
Currently, biosensors are made with E. coli because it naturally colonizes the mammalian gut and many genetic engineering techniques have already been developed for this species. Unfortunately, E. coli does not live in the honeybee gut so designing a biosensor for this species required Chhun and colleagues to start from scratch.
Step one was identifying which honeybee gut bacteria are most amenable to genetic engineering. Initial experiments revealed Snodgrassella alvi (S.alvi ) as the best candidate. Next, the synthetic biology techniques used to add genes to bacteria had to be adapted and optimized for S. alvi.
One common technique for adding genes to bacteria is via a plasmid. In bacteria, DNA is exists in circular units called plasmids. Researchers insert new plasmids into a bacterium which contain the new genes, or in this cast the circuit of genes, they want the bacteria to express.
To test whether a S. alvi biosensor could work and survive in honeybees, Chhun inserted a plasmid containing a simple genetic circuit that detects a sugar derivative called isopropylthio-β-galactoside (IPTG) and which produces a green fluorescent protein in response. Chhun chose IPTG as the target because they could add varying concentrations of it to the honeybee’s food and measure how much, if any, fluorescent protein was produced.
But there was one problem: getting bees to defect in the lab.
Honeybee massages
To see the changes in fluorescence produced by the sensors, researchers working with mammals simply collect stool samples. However, with bees, this is not so simple due to the insects’ impressive hygiene.
As Chhun explained, bees always leave the hive to defecate. “So, when we keep them in the lab in cages, for them this is the hive,” said Chhun, “and because they don’t go outside, they don’t defecate, they just hold it in.”
Faced with a lab full of constipated bees, Chhun and his colleagues turned to a low-tech solution: massage. “If you just massage them, then they will defecate,” he said.
To massage them without getting stung, they leveraged the insect’s inability to resist cold temperatures. “If you put them on ice, they fall asleep,” said Chhun, “then we just massage the abdomen and make them defecate into a tube.”
Fluorescent bee feces
The massaging worked and there was a detectable change in fluorescence in the feces corresponding to an increasing concentration of IPTG in the honeybee’s food.
This is an exciting proof of concept but according to Chhun, there are still some tweaks required. “One of the things we could improve is that this biosensor system is leaky,” he said, meaning even in the absence of IPTG, there was always some fluorescence. “If we want to study other molecules, especially maybe low concentration of a molecule, we need to tweak the system so that the basal level of expression is very, very low,” he said.
With this first step achieved, the team is now working to design S. alvi sensors that detect other molecules microbiome researchers are interested in. Eventually he aims to design sensors with memory too. “There’s a lot of molecules in the gut that are transient,” said Chhun, “and we want our sensor to be able to tell us, okay, this was five days ago, but five days ago, there was this molecule.”
With such a tool, microbiome researchers can start to track how the microbiome is communicating with the host and influencing health. Biosensors could also serve as monitors of for honeybee health and conservation, although ethical discussion about introducing engineered bacteria to wild bees are a long way from being resolved.
Reference: Audam Chhun, et al. An engineered bacterial symbiont allows noninvasive biosensing of the honey bee gut environment, PLOS Biology (2024), DOI: 10.1371/journal.pbio.3002523
Feature image credit: Aduam Chhun, et al.