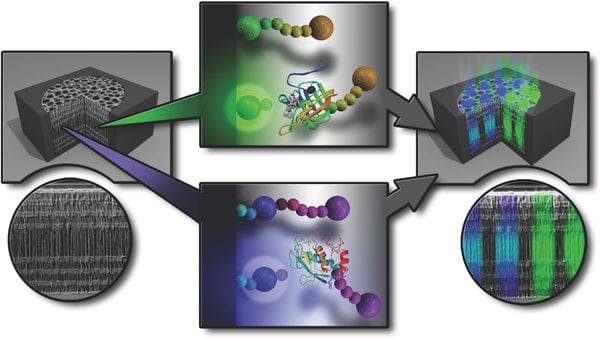
Researchers from the University of South Australia are featured in Advanced Science for developing a biosensor to detect Sortase A (SrtA) in chronic wounds. Chronic wounds are considered to be a widespread health issue today and are costly to treat. Staphylococcus aureus (S. aureus) bacteria found in chronic wounds can cause infection, leading to potentially life-threatening complications. Therefore, devices with the ability to indirectly detect and monitor S. aureus at the point of care allow for early diagnosis of infection. The SrtA enyzme plays a key role in the virulence processes of S. aureus and can serve as a biomarker for infection caused by this bacteria. The mechanism responsible for virulence involves cleavage by SrtA of the threonine-glycine bond in the LPXTG amino acid motif of surface proteins to form linkages with the bacterial cell wall.
 The biosensor is based on a porous silicon resonant microcavity (pSiRM) modified with a fluorescence resonance energy transfer (FRET) peptide. The peptide substrate is covalently bound to the surface of the pSiRM by an LPETG amino acid sequence tethered to fluorescein isothiocyanate (FITC) as a fluorescent dye, 2,4-dinitrophenol (Dnp) as a quencher, and an amino group. When exposed to SrtA, the peptide is cleaved, leaving FITC bound to the piSIRM and emitting a fluorescence signal that can be detected by either a fluorometer or confocal microscope. In bacterial culture medium inoculated with S. aureus, the biosensor was capable of detecting SrtA after only 0.5 hours. SrtA was also detected in samples of human wound fluid inoculated with S. aureus for 24 hours; the concentration of SrtA in the wound fluid was lower compared to 24-hour inoculation in bacterial culture medium. The biosensor was not only capable of detecting SrtA in human wound fluid but could also distinguish between two different enzymes in the wound environment. When MMP (metalloproteinase) peptide substrate was immobilized on pSiRM along with SrtA peptide substrate, SrtA selectively cleaved the SrtA peptide substrate, demonstrating the biosensor’s multiplexed sensing capability.
The biosensor is based on a porous silicon resonant microcavity (pSiRM) modified with a fluorescence resonance energy transfer (FRET) peptide. The peptide substrate is covalently bound to the surface of the pSiRM by an LPETG amino acid sequence tethered to fluorescein isothiocyanate (FITC) as a fluorescent dye, 2,4-dinitrophenol (Dnp) as a quencher, and an amino group. When exposed to SrtA, the peptide is cleaved, leaving FITC bound to the piSIRM and emitting a fluorescence signal that can be detected by either a fluorometer or confocal microscope. In bacterial culture medium inoculated with S. aureus, the biosensor was capable of detecting SrtA after only 0.5 hours. SrtA was also detected in samples of human wound fluid inoculated with S. aureus for 24 hours; the concentration of SrtA in the wound fluid was lower compared to 24-hour inoculation in bacterial culture medium. The biosensor was not only capable of detecting SrtA in human wound fluid but could also distinguish between two different enzymes in the wound environment. When MMP (metalloproteinase) peptide substrate was immobilized on pSiRM along with SrtA peptide substrate, SrtA selectively cleaved the SrtA peptide substrate, demonstrating the biosensor’s multiplexed sensing capability.