Lithium ion batteries (LIBs) are a ubiquitous energy technology, but their high cost is an issue for stationary applications. Owing to their earth-abundance, sodium ion batteries (NIBs) are cheaper alternatives to LIBs. The size difference between sodium and lithium ions, however, means that new electrode materials must be developed in order for NIBs to be viable. Of those reported to date, perovskite-type NaFeF3 has been demonstrated to be a suitable positive electrode material, but drawbacks including poor reversibility and low capacity have so far limited its practical application.
Prof. Nicola Pinna from Humboldt University of Berlin and co-workers present an NaFeF3 electrode capable of fully reversible insertion of both lithium and sodium ions.
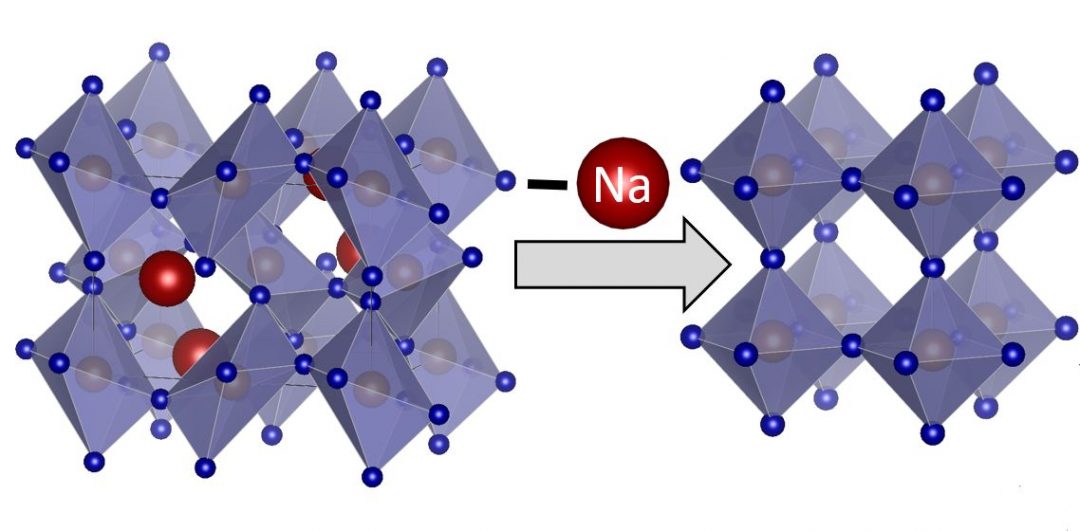
NaFeF3 was synthesized by first preparing nanoscale FeF2 colloids through the fluorination of iron(II) chloride. Oleylamine was also added in this step in order to stabilize the nanosized particles. The oleylamine-stabilized FeF2 was then reacted with sodium ethoxide and ammonium fluoride under microwave irradiation for 5 minutes in an inert atmosphere to obtain nanosized NaFeF3.
The performance of NaFeF3 as a cathode for NIBs was evaluated using cyclic voltammetry, revealing excellent capacity retention up to 60 cycles with 99% coulombic efficiency after five cycles. Unexpectedly, NaFeF3//Li half-cells could be reversibly cycled through a full insertion mechanism of one Li per Fe with no decomposition. After complete Na deinsertion, reversible Li insertion occurs with a capacity retention above 200 mAh g–1 after 50 cycles at a rate of C/3 and 180 mAh g–1 after 100 cycles at 1C at high scan rates.
This result is unprecedented for fluoride materials, and can be explained by a kinetically controlled mechanism that suppresses the trirutile phase transition.