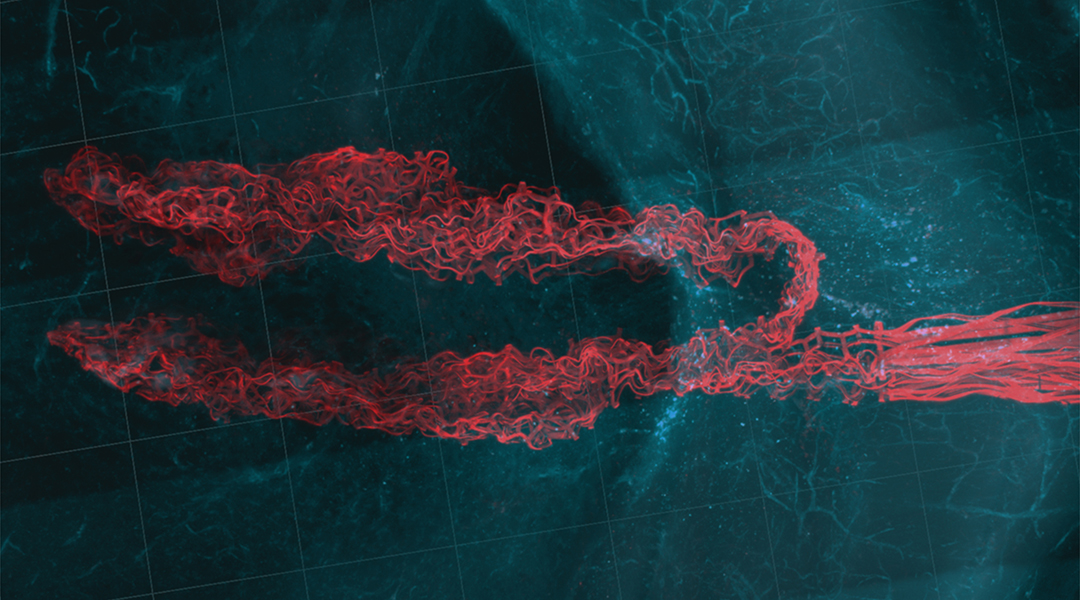
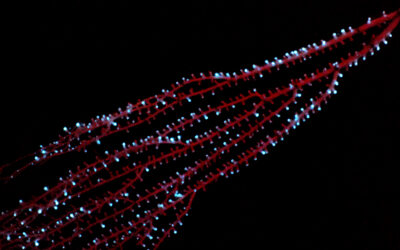
One of the most rapidly developing areas of medicine is neuroscience — the investigation of the inner workings of the brain, an area of the body that is notoriously difficult to probe. The understanding of how neuronal networks function is vital to our understanding of neurological diseases, such as Alzheimer’s and Parkinson’s, as well as issues such as depression and addiction, making this a pressing area of scientific investigation.
Neuronal networks can be monitored using probes that are capable of recording electrical signals from the individual neurons that comprise these networks. These electrical signals constitute communication between neurons, so monitoring them can grant scientists insight into how different regions of the brain — and, crucially, how disease and other factors — affect and disrupt this communication. Monitoring can also be used to assess the effects of drugs used to treat these brain ailments.
Currently, these investigations are conducted using the implantation of polymer-based and silicon probes, but these devices suffer from limitations that hinder their performance and make them overly intrusive. This includes the fact that reaching different regions of the brain requires implanting more and more “traditional” probes, each with its own interface.
These probes can be cumbersome, adding weight to the system and increasing the risk of probes slipping in relation to their counterparts, something that can decrease the system’s capability to monitor neural networks. These issues could be eliminated if a neuronal monitoring system could be created that uses a single interface and employs lightweight probes with a thread-like structure.
A thread-like, flexible probe
Creating just such a system was the aim of Charles M. Lieber, Lieber Research Group and retired professor from Harvard University with Jung Min Lee of Korea University, who was a postdoctoral fellow with Lieber. Lee is the lead author of a paper published in the journal Advanced Science that details the neuronal probe.
“This platform addresses the issues with implantable neural probes by utilizing a ‘thread-like’ flexible and biocompatible single probe that can be implanted into two or more sites using glass or thin metal needles, where the flexible and biocompatible properties ensure a seamless and stable interface to the brain tissue,” said Lieber. “Additionally, this platform allows for monitoring neural activities from multiple distinct brain sites, which may be widely separated, using a single low-mass interface.”
He adds that this is an improvement over multiple single-shank probes that are limited by the sizes and weights of interfaces to multiple probes and suffer from an ability to track changes in neuronal activity over longer time periods.
The team based their thread-like probes on previous mesh electronics they had developed, which allowed them to create a three-layer “sandwich” of two layers of polymer separated by metal. The polymers serve to insulate the metal conductors from the brain and make the probe biocompatible with the tissue surrounding it.
The material selected by the team is a commercial polymer called SU-8 which can be made into a viscous polymer that can spun as thin as small as 1 micron (about 0.0001 cm), or can be stacked to create laminates of above 1 mm thick.
Lieber explained that SU-8 can be used directly in photolithography, a patterning process used to make the probes in which a photosensitive polymer is exposed to light in selected areas, leaving an image that can be patterned across an underlying substrate. SU-8 is also stable over long periods of time.
“Another key feature of our designs is the sub-micron thickness of the polymer and metal layers. This thinness, together with the flexible properties of SU-8 polymer, gives implants that have similar flexibility to the brain tissue itself,” explained Lieber. “This matching of flexibility is a key part of the stability in the brain and the fact that there is minimal adverse reaction upon implantation.”
Visualizing the brain’s networks
The researchers tested the devices by implanting them into two or four sites in semi-transparent hydrogels, a material composed of a 3D network structure able to store large amounts of water, which was selected thanks to the fact it has similar mechanical properties to brain tissue.
“This allowed us to visualize and optimize the multi-site stitching and show that we could achieve a high yield of electrical conductivity even when sewing the thread-like probe,” Lieber continued.
The team then conducted in vivo studies using mice that showed them that every site of the stitched probes had a seamless tissue-probe interface, creating a negligible adverse immune response from the tissue.
“We were actually quite surprised at first to see that stitching our probes in single probes in two or even four sites yielded exactly the same reliability as we found previously for probes implanted in only one site — that is, seamless probe tissue interfaces and stable recording of individual neurons from electrodes in each of the regions over at least a month’s time,” Lieber added. “This has thus enabled us to think boldly about future opportunities.”
The authors said they are now working towards increasing the number of regions and the total number of neurons monitored, incorporating the potential to monitor and modulate neuron and network activity through stimulation.
“The field of electronic neural implants stands on the brink of a new era, encompassing fundamental research, therapeutic interventions, and various applications of medicine,” Lieber concluded.
References: J., M., Lee., et al., Stitching Flexible Electronics into the Brain, Advanced Science, (2023), DOI: 10.1002/advs.202300220