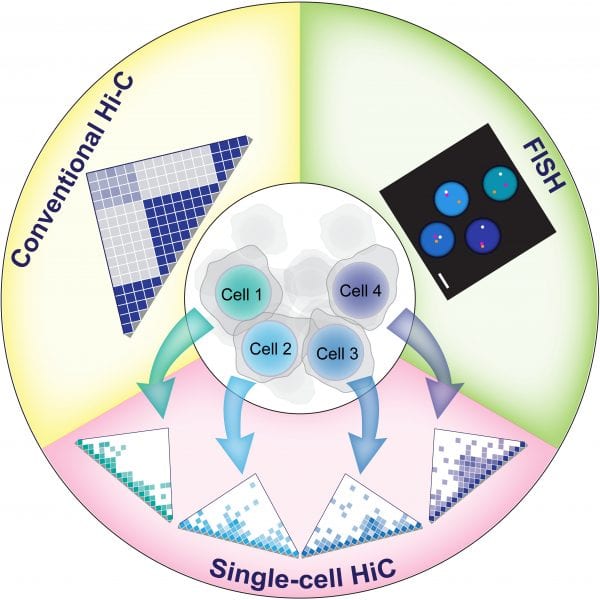
Chromatin folding is a dynamic process, and this dynamic nature most likely is also important for major genetic processes. Because the analysis of an entire cell population will mask any differences found between individual cells, being able to analyze the chromatin spatial organization of individual cells is an important step forward. FISH (fluorescence in situ hybridization) already provides a good possibility to study physical distances between individual genomic locations in single cells. However, with this technique normally only a limited number of loci can be analyzed at the same time.
Recent advances in single cell techniques have now made it possible to analyze chromosome conformation in a large number of individual cells simultaneously. This will now also allow the study of chromatin folding in rare cell types such as stem cells, tumor progenitors, oocytes and totipotent cells that might not be obtained in numbers sufficient enough for a conventional Hi-C experiment.
In their review published in BioEssays, Sergey Ulianov et al. compare and discuss different strategies for single cell Hi-C analysis. These different studies have for example revealed a prominent cell-to-cell variability with regards to both inter- and intra-chromosomal contacts of X chromosome domains. The use of these new single cell Hi-C techniques in combination with other methods such as 3D FISH, live cell imaging and single cell RNA-seq will provide further insights into the inner working of the nucleus and its 3D genome organization.
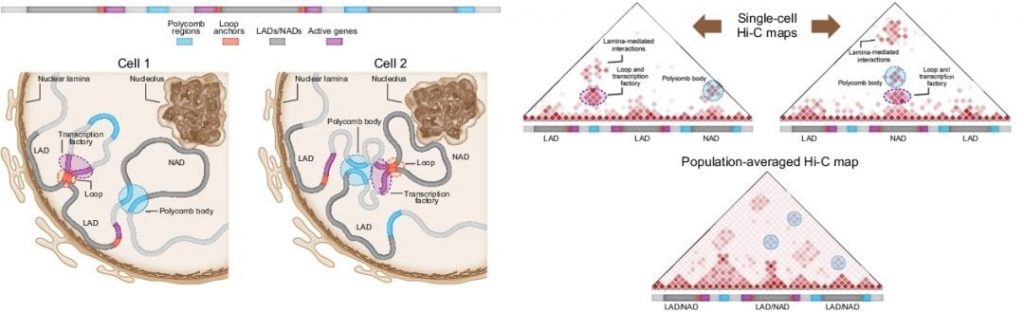
Schematic showing the differences in chromatin organization between two individual cells and the corresponding single cell Hi-C maps as well as a population-averaged Hi-C map. LAD: lamina-associated domain; NAD: nucleolus-associated domain.