Functional polymers, especially water-soluble ones, have found numerous applications in the 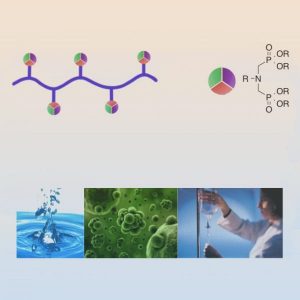 biomedical field. However, synthesis of such polymers can often be challenging due to the incompatibility of the functional groups with many polymerization methods. Therefore, it is critical to explore new routes to such functional materials.
biomedical field. However, synthesis of such polymers can often be challenging due to the incompatibility of the functional groups with many polymerization methods. Therefore, it is critical to explore new routes to such functional materials.
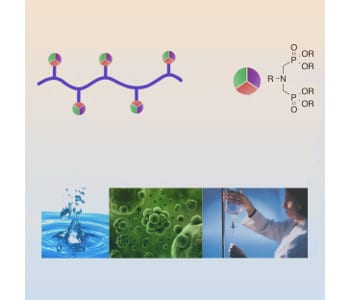
Multicomponent reactions are a class of extremely versatile reactions that can introduce multiple functionalities in a single, one-pot reaction. Combining this functionalization method with controlled polymerization techniques offers promising opportunities to create well-defined functional materials. Aminobisphosphonates are attractive compounds in many different fields especially due to their chelating ability. The aminobisphosphonate moiety creates a chelating group that offers three binding sites, improving the overall stability of the binding complex. This chelating ability can be utilized in a variety of ways, ranging from water purification to biomedical applications
Researchers from the University of Florida describe the synthesis of well-defined polyacrylamides bearing aminobisphosphonate groups on nearly every repeat unit by using controlled radical polymerization and a post-polymerization multicomponent reaction. The reactions were optimized to increase the functionalization efficiency to create water-soluble, highly ionized materials.
Due to their binding ability, these polymers can chelate radionuclides, which are used for internal radiation therapy. Moreover, phosphonic acid groups are known to target bone tissue, making these compounds potentially useful in the treatment of bone cancer. The group ultimately envisions a combination of active targeting through the phosphonic acid groups and passive targeting through the enhanced permeation and retention (EPR) effect, a well-studied phenomenon showing exclusion of macromolecules from healthy tissue and preferential accumulation in cancer cells.