Soap is made from classical amphiphilic molecules which contain a hydrophobic part and a hydrophilic part, which together dissolve oil and grease. Amphiphiles are often surface active (accumulate at interfaces) and form multi-molecular, spherical aggregates (micelles) in water. In the classical micelle, hydrophobic parts avoid contacts with water and fill the micellar core. Inversely, hydrophilic parts are exposed towards bulk water and form the micellar shell.
Mikael Lund, Pavel Matějíček, and colleagues (from University of Lund, Charles University Prague, National University of Science and Technology Moscow, and Czech Academy of Sciences Prague) in their present work have studied a special amphiphile, COSAN, which does not follow the classical paradigm.
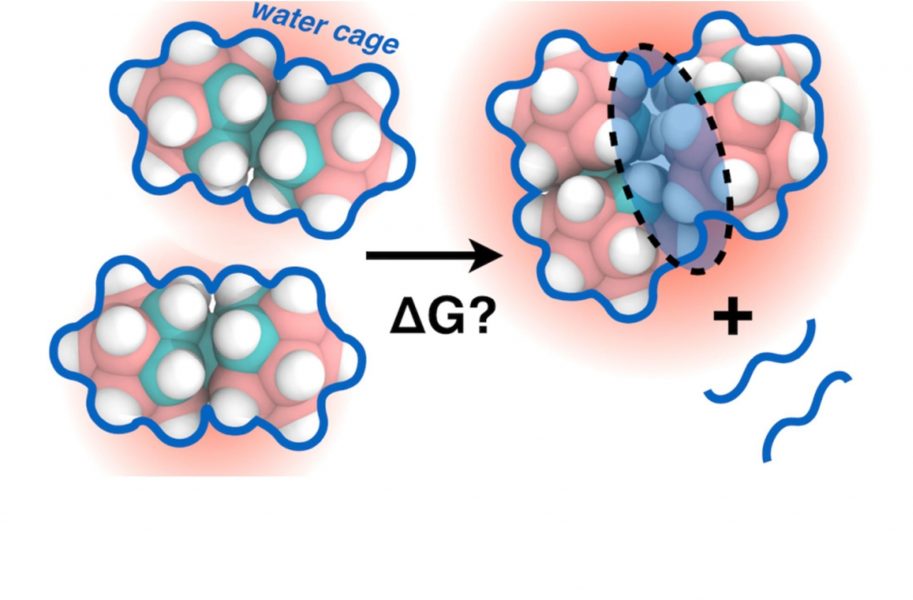
COSAN belongs to the anionic boron cluster compounds with delocalized negative charge. It has a polyhedral shape consisting of B–H units (pink–white spheres in the image above), where four of them are replaced by C–H groups (cyan–white spheres). COSAN behaves like a classical amphiphile in many ways (it forms micelles in water), however, it has a non-amphiphilic structure—there are no distinct hydrophobic and hydrophilic regions.
The paper describes a combined experimental and theoretical study employing NMR spectroscopy, calorimetry, molecular dynamics (MD) and quantum chemical calculations which provide an understanding of the driving force of the self-assembly of COSAN molecules. The hydration shell of COSAN, obtained by MD simulations, resembles a patchy network with slightly hydrophilic spots close to the C-H segments, giving rise to a strong hydrophobic self-association. The simulations further give the counter-intuitive result that two like charged ions can attract each other.
In summary, this case study of COSAN self-assembly in water provides a detailed understanding of micellization driven by a non-classical hydrophobic effect.