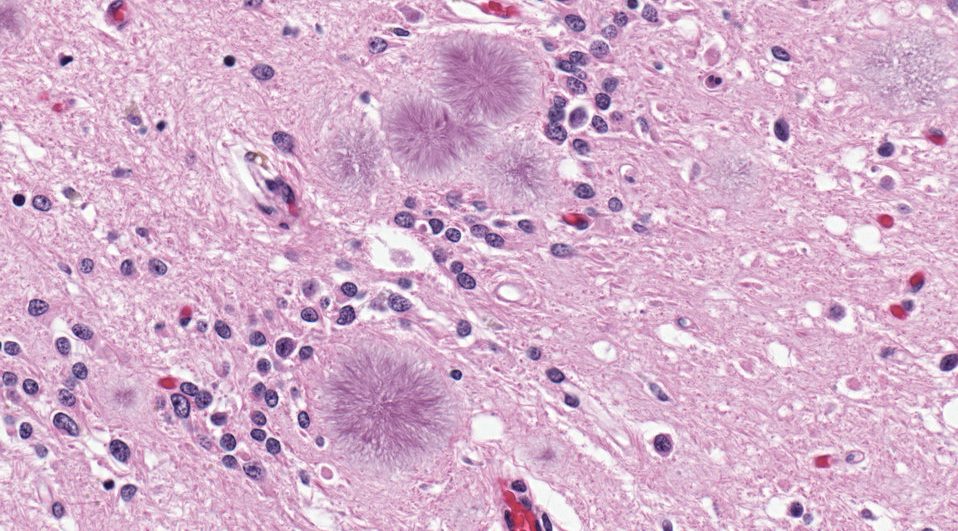
Amyloids, perhaps most famous for their association with diseases such as Alzheimer’s, have recently been recognized as biological structures that perform vital physiological functions in their host organisms. A recent review in Peptide Science by Soultan Al-Halifa and colleagues specifically discusses the potential applications of amyloids in nanovaccine engineering and biosensing.
Amyloids, which are highly organized proteinaceous structures, have unique mechanical, physical, and biological properties relating to their structure. Al-Halifa and colleagues detail some of these properties, including spontaneous self-assembly of polypeptide chains, high mechanical resistance, ability to modulate the structure and morphology of the amyloid, biocompatibility and biodegradability, and high thermal, chemical, and enzymatic stability.

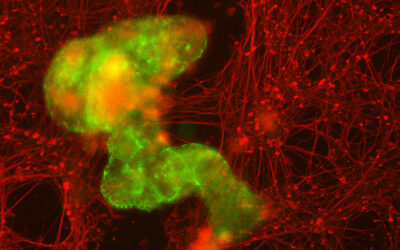
A. An image of amyloid fibrils. B. A schematic representation of an amyloid fibril.
The biocompatibility and biodegradability along with the stability of amyloid structures makes them ideal candidates for nanovaccines. Nanovaccines can trigger immune responses without the shortcomings of traditional vaccines, such as return of inactivated viruses to virulence, weak immune response, or allergic reactions to the vaccine vector. Using self-assembling amyloid structures as the platform for nanovaccines takes advantage of the stability of amyloids, which are resistant to proteolysis. Amyloid structures can also carry multiple copies of antigenic determinants, thereby creating a stronger immune response.
The field of nanotechnology also makes use of amyloid biocompatibility and biodegradability. Additionally, modifications to the monomeric peptide sequences within the amyloid structure can lead to improved control over the physical, chemical, and mechanical properties of the final amyloid structure. Ideally, amyloid assemblies (functionalized with appropriate molecules) can be used to study biological events at the nanoscale level.
Despite the association of amyloids with human diseases, the review by Al-Halifa and colleagues details exciting work studying the opportunities for positive use of these folded protein structures. Though challenges still face the widespread application of amyloid structures, including the fact that it is still impossible to maintain precise control over amyloid growth, the authors remain positive. They state that with various scientific fields all examining the issues facing amyloid structure use, these issues will be resolved for the application of amyloid structures in multiple fields, including nanovaccines and biosensing.