According to a 2007 US national survey, roughly 1.7 million hospital-acquired infections are reported annually, accounting for more than 99,000 deaths, with roughly half attributed to the growth of bacterial biofilms.
These are complex protein matrices that provide “survival sites” for pathogenic bacteria, protecting them from the body’s defense mechanisms and reducing the diffusion rate of antimicrobials, making the bacteria more resistant.
“Our goal is to reduce infection and complications from implanted medical devices,” explained Amir Sheikhi, professor at the Department of Chemical Engineering, Pennsylvania State University, in an email to ASN. “Antibiotics are typically used to treat these complications. However, microbes have been evolving resistance to antibiotics over time to spawn ‘superbugs‘. We need to maintain global antimicrobial stewardship by seeking ways to prevent infection without the overuse of antibiotics.”
Sheikhi and his collaborators, which include Richard Kaner at the University of California, Los Angeles and Ali Khademhosseini, director and CEO of the Terasaki Institute, propose a promising antibacterial surface treatment that resists protein and bacterial adhesion.
Zwitterionic antibacterial surface
The approach involves depositing a zwitterionic material on the surface of devices, such as catheters, stents, heart valves, and pacemakers, which prevents bacteria and other harmful materials from binding to its surface. “Zwitterions are a class of materials that contain equal positive and negative charge within its structure so that the overall charge of the material is neutral,” explained Sheikhi. “Many biomaterials are zwitterionic in nature such as amino acids — the building blocks of proteins, and phospholipids — which make up the cellular membrane bilayer.
“Zwitterionic materials themselves do not kill microbes,” he added. “They simply prevent microbes from depositing and proliferating as biofilms on surfaces.”
The benefits of antibacterial zwitterionic surface treatments have been known for many years. However, they have not translated into clinical applications due to the fact that current materials require complex reaction conditions and expensive reagents. But this is where the current study shines.
To begin, the team chose the zwitterionic polymer polysulfobetaine due to its biocompatibility, its antibacterial properties, and stability. The substrate can be permanently bound to device surfaces using UV light and a molecular anchor under ambient conditions. As a result, polysulfobetaine can be used to rapidly coat a broad range of materials with no preconditioning steps, making wide-scale manufacturing accessible.
“We have developed a simple zwitterionic treatment chemistry and deposition technique that enables us to apply the material to common medical devices with known complications,” said Kaner. “Catheters, for example, are one of the most common medical devices used in healthcare and are fraught with complications, such as catheter-associated urinary tract infection, occlusion, pain, and swelling.”
Clinical tests
In lab tests, the team tested their antibacterial surface on modified devices and tested their resistance to different bacteria, fungi, and proteins, and found that the treatment reduced biofilm growth by more than 80%. In a clinical setting, 16 long-term urinary catheter users switched to FDA-approved silicone catheters with the new zwitterionic surface treatment. Ten of the patients described their urinary tract condition using the surface-treated catheter as “much better” or “very much better,” and 13 chose to continue using the new catheter over conventional latex and silicone options after the study period ended.
“After demonstrating the biocompatibility and safety of the device, the catheters are now commercially available for use in the clinical setting,” said Sheikhi. “The main hurdle to overcome is the status quo. Many of these commodity devices have been used for decades, and antibiotics are often co-administered to reduce complication rates.
“Convincing these large, ossified institutions that they should modify their standard of care with superior technology is always challenging,” he continued. “We are continuing to collect more data to demonstrate that not only will the treated devices improve the lives of their patients, but back-end costs will be drastically reduced due to the lower complication rates and less required intervention to treat the complications.”
Reference: Amir Sheikhi, Richard B. Kaner, et al., A Readily Scalable, Clinically Demonstrated, Antibiofouling Zwitterionic Surface Treatment for Implantable Medical Devices, Advanced Materials (2022). DOI: 10.1002/adma.202200254
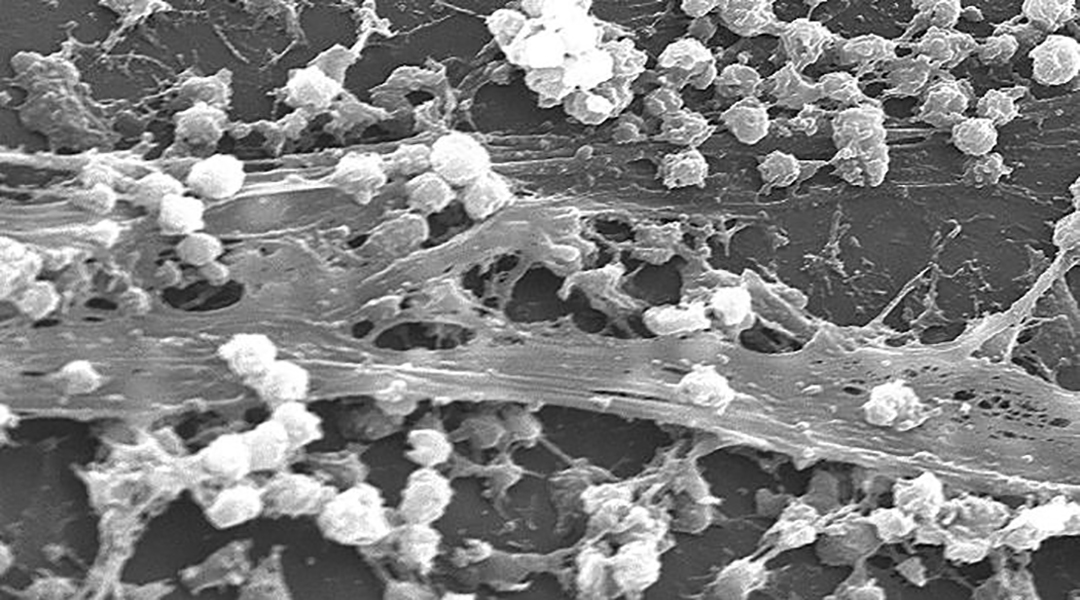
Feature image: Scanning electron microscopic (SEM) image depicting large numbers of Staphylococcus aureus bacteria, which were found on the luminal surface of an indwelling catheter. Credit: Janice Haney Carr/CDC