With the help of a llama, researchers have devised a new strategy to combat HIV type 1. Unlike humans, llamas are a source of virus-targeting entities called nanobodies, which, when combined with a specific human antibody, can lower the virus’ chances of escaping neutralization.
“HIV-1 is a very challenging virus, and our plan was to repetitively immunize animals over a relatively long period of time to obtain neutralizing nanobodies,” said Jianliang Xu, one of the researchers who headed the study and an assistant professor of biology at Georgia State University.
Since 1981, when the first case of HIV was reported in the United States, a vaccine has remained beyond reach for a few reasons, including the rapid mutation of the virus, which leads to treatment resistance.
Antibodies that are effective against multiple HIV-1 strains, known as broadly neutralizing antibodies, are a promising treatment option, but they still need to be stronger and better designed to maintain their efficacy against virus mutation.
They can also take years to develop as human antibodies, which consist of heavy and light amino acid chains, cannot easily maneuver around the natural defenses the virus has set up to reach their target binding sites on the virus’ surface.
Nanobodies, small fragments of antibodies, could offer a solution to this predicament.
Llamas may hold the key to an HIV treatment
Camelid animals, including camels, alpacas, and llamas, produce a specific type of antibody known as heavy-chain-only antibodies, which are the main source of nanobodies, Xu explained.
“Nanobodies are about ten times smaller than conventional antibodies,” he stated. “They have an [elongated] shape and are good at binding smaller, often partially occluded sites on [HIV-1] antigens, which may be inaccessible to classical [human] antibodies.”
Nanobodies neutralize HIV-1 antigens by binding to the envelope protein spike, which the virus uses to enter healthy host cells, thus shutting down infection. To obtain the nanobodies, the scientists first needed to induce the production of heavy-chain antibodies in a camelid animal. To do this, Xu and his colleagues injected a single llama with an HIV-1 antigen — the part of the virus that the immune system recognizes as invasive, prompting it to respond.
The HIV-1 antigen they used was fixed in a “closed” conformation with well-exposed target sites, making the immune system more likely to detect the virus. In response, the llama, immunized 13 times over the course of 271 days, produced blood cells that generated heavy-chain-only antibodies from which nanobody genes could be isolated.
These genes allowed the researchers to produce large quantities of the nanobodies in a controlled environment using a technique called phage display, in which the nanobody genes were inserted into viruses that infect bacteria, called phages. These phages displayed the nanobodies on their surface, allowing them to bind HIV-1 antigens.
“We used different HIV-1 protein antigens to select for phages that displayed antigen-binding nanobodies on their surface,” Xu said.
Among the numerous nanobody candidates isolated from the llama’s blood, the researchers identified those that were most effective against a range of HIV strains and had the strongest ability to neutralize the virus.
Two antibodies are better than one
Although the best nanobodies demonstrated promising results on their own, the researchers aimed for even better neutralization performance. According to Peter Kwong, a professor of biochemistry and molecular biophysics at Columbia University and collaborator on the study, the goal for HIV-1 neutralization is to achieve 100% neutralization at the lowest possible concentration.
“For a therapeutic, it is desirable to inhibit many, if not all, virus strains, enabling close to complete viral suppression in AIDS patients,” he commented.
Even though a single broadly neutralizing antibody or nanobody may be able to neutralize most or even all HIV-1 strains, new mutants will continue to be generated owing to the instability of the virus genome, Xu explained. Simply by chance, some mutants undergo subtle changes that allow them to escape antibody or nanobody binding.
“Treatment with one antibody targeting a single site usually leads to the accumulation of virus mutants that are resistant to the antibody,” he said.
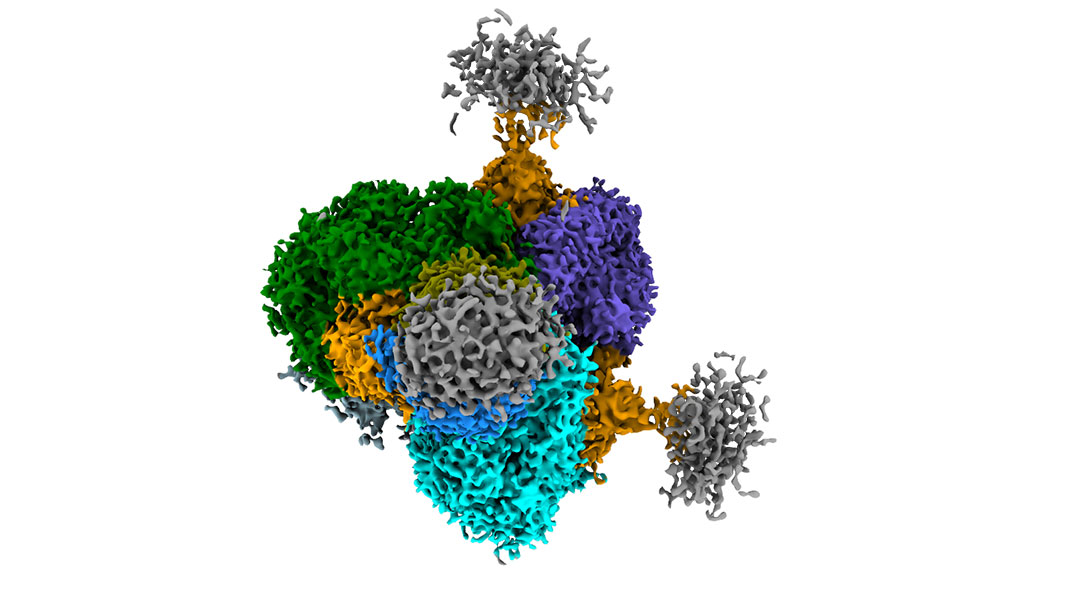
Building on their previous work, Xu, Kwong, and their team engineered a human–llama “chimera” or bispecific antibody that can bind to two of the virus’ vulnerable sites simultaneously, making it much more difficult for the virus to evade neutralization.
This two-pronged attack is possible owing to the different shapes and binding preferences of the llama nanobody and human antibody, which were linked together. The nanobody binds to a specific site known as the CD4 binding site, while the human antibody targets another site known as the V2 apex.
Not only was a very small concentration of the human–llama antibody needed to neutralize the HIV-1, but it neutralized more than 95% of 208 HIV-1 strains simultaneously, making it ten times more potent than the human–llama antibody the researchers previously engineered.
Feasibility for HIV-1 prevention and treatment
Although these results are promising, more practical aspects need to be taken into account, including the antibody’s half-life, or the time required for its concentration to diminish by half after injection in the human body.
“Half-life is important, as [preventative] treatment would benefit from an antibody that only needs to be dosed once a year,” Kwong stressed, making any therapy based on this technology more easily accessible.
“Critically, we’ve observed that the half-life of the [human antibody]–nanobody is substantially higher than the nanobody by itself,” he added. This is because intravenously injected nanobodies, owing to their small size, are typically cleared from blood serum within an hour by kidney filtration. Connecting it with a human antibody adds bulk, slowing its clearance. The researchers are currently testing the half-life of a variation of the bispecific antibody reported in their paper.
The researchers also immunized only one llama. At this stage, it’s unclear if immunizing more llamas would lead to broader HIV-1 neutralization, or if other animals could produce more potent nanobodies.
“Another limitation is that all strong neutralizing nanobodies we isolated are targeting the CD4 binding site,” Xu pointed out. “It would be ideal if we could obtain broad and potent neutralizers that recognize other sites on the HIV-1 envelope protein. That way we could build bispecific or multi-specific antibodies with more combinations.”
In addition, the HIV-1 antigen used in the study mainly induced an immune response towards a non-neutralizing site of the antigen, particularly its base.
“This probably could be avoided if we use immunogens that have the base region genetically covered,” Kwong said. The researchers hope to address this issue in future studies.
Sharana Mahomed, a clinical microbiologist at the Centre for the AIDS Programme of Research in South Africa (CAPRISA) and the University of KwaZulu-Natal, South Africa, who was not involved in the study, said that these study results are promising. Her research mainly focuses on the use of broadly neutralizing monoclonal antibodies against HIV, and she’s currently running clinical trials aimed at fighting the infection in sub-Saharan Africa.
“[Xu and Kwong’s] strategy provides extensive structural and functional insights into the bispecific antibodies’ mechanisms, achieving exceptional neutralization potency and breadth, thus highlighting their clinical relevance,” Mahomed commented. “Furthermore, the study provides detailed structural and functional insights into how these antibodies target the virus, offering a mechanistic understanding that can guide future antibody design.”
“However, the lengthy immunization period presents scalability challenges, and the species-specific responses of llama-derived nanobodies may differ in humans, necessitating further validation,” she noted.
She told us that overcoming these challenges will not only require validation in humans but faster immunization techniques, scalable manufacturing processes, cost-effective production, and strategic partnerships for equitable distribution.
Despite the remaining obstacles, the newly developed antibody represents progress in the now decades-long effort to develop an HIV-1 treatment that can effectively inhibit all strains of the virus.
Reference: Jianliang Xu, Peter D. Kwong, et al. Ultrapotent Broadly Neutralizing Human-llama Bispecific Antibodies against HIV-1, Advanced Science (2024). DOI: 10.1002/advs.202309268