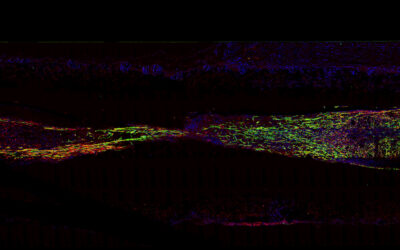
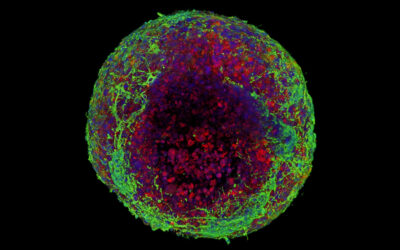
The heart is an incredible organ. Through a coordinated effort, the heart’s four chambers and connecting valves work in unison to regulate blood flow, keeping oxygen and nutrients circulating throughout the body.
“It is fascinating to learn about [heart valves’] exquisite balance of properties, which enable them to withstand millions of opening and closing cycles in their lifetime,” said Petra Mela, professor in the Department of Mechanical Engineering at the Technical University of Munich. “How can the native heart valves achieve this? What sort of material are they made of to provide these outstanding features so perfectly well?”
Mela and her collaborator Elena De-Juan-Pardo of the Harry Perkins Institute of Medical Research and the University of Western Australia have been developing a means to create artificial heart valves derived from a patients’ own cells that go beyond just artificial prosthesis.
“The main drive for us is the potential impact of our work on patients worldwide,” said De-Juan-Pardo. “Heart valve disease is the third leading contributor to cardiovascular disease, which is the number one cause of mortality worldwide.”
If repairing a broken-down valve is not possible, current standard of care involves implanting a prosthetic valve. “The prosthesis should ideally remain in the patient for the whole lifetime,” said De-Juan-Pardo. “Yet that valve will only last a limited number of years.”
This means that patients must undergo multiple interventions as the artificial valves wear out or, in pediatric patients, as new valves are required to match the valve size with their growing bodies. Available mechanical and biological heart valves are inherently unable to remodel and grow with the child, say the researchers.
“We are committed in solving these issues by bringing the latest technological advances in 3D printing to the next level to manufacture artificial heart valves with the ability to grow and remodel with the patient, offering a regenerative approach as opposed to a permanent implant inherently unable to adapt,” said Mela.
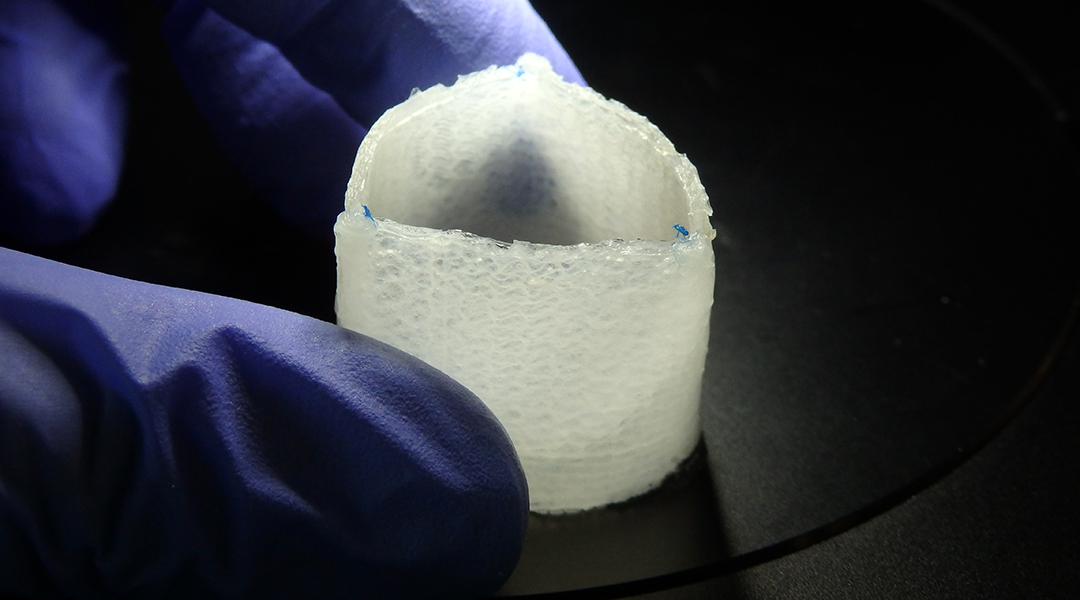
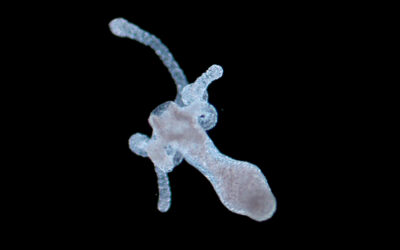
In their study published in Advanced Functional Materials, Mela, De-Juan-Pardo, and their teams used a 3D-printing technique called melt electrowriting to create heart valve implants with biologically inspired structural heterogeneity, potentially endowed with the ability to grow with the patient.
“Melt electrowriting lends itself very well to tissue engineering as it allows us to exquisitely arrange the deposition of the fibers into complex patterns,” said De-Juan-Pardo. “This enables us to mimic the orientation of some of the structural components seen in soft tissues, in this case the aortic heart valve.”
In contrast to conventional 3D printing, melt electrowriting combines an applied electric field, temperature, and pressure to create a charged jet of molten polymer. This allows researchers to deposit microfibers that are ~1/10 the thickness of a human hair with incredible precision in a predefined pattern. “This results in scaffolds with excellent features,” added Mela.
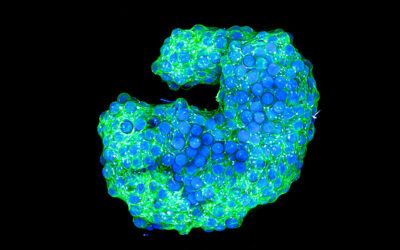
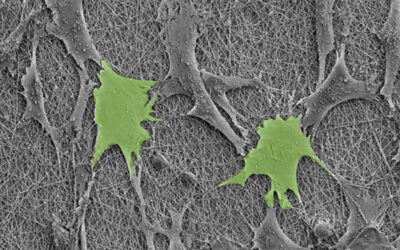
The challenge, say the team, was manufacturing a scaffold that is able to withstand the demanding functions of a heart valve while also remaining porous enough to allow the patient’s own cells to colonize the scaffold and thrive.
“In fact, the sub-optimal structural properties of dense fibrous scaffolds to date are a major issue jeopardizing their clinical potential,” said Mela. “By using melt electrowriting’s extraordinary accuracy and a custom-made digital platform, we demonstrate a pioneering proof-of-concept for an off-the-shelf complete heart valve construct that could further be extended to other soft tissue engineering applications.”
The team’s digital platform is designed to manufacture complex patterns that mimic the various tissue structures observed throughout the heart valve, which are then composited with micro-porous hydrogel scaffolds. “Together these provide both the structural integrity required to withstand the demanding cardiovascular loading conditions and adequate porosity to allow cell infiltration,” added De-Juan-Pardo. “The result is a one-of-a-kind tubular heart valve construct, benefiting from both classes of scaffolds.”
To test the capabilities of their artificial heart valves, the researchers created a mock circulatory system and subject them to the same pressure and flow rates that a natural heart valve would experience. The results were promising, demonstrating excellent performance under a number of conditions, satisfying the industry standards.
Further testing is required before the heart valve constructs can be used in a clinical setting. After ensuring the material is durable enough, it will be tested in animal models in a pre-clinical setting. “The main challenges here will be understanding and optimizing the implantation method, the analysis of the biological response triggered by the valve and its hemodynamic functionality for an extended time in large animals,” said Mela. “After successful long-term animal studies, we would be in the position to start preparing for the first human clinical trials before moving to treating patients regularly.”
Reference: Navid Toosi Saidy, et al., Spatially Heterogeneous Tubular Scaffolds for In Situ Heart Valve Tissue Engineering Using Melt Electrowriting, Advanced Functional Materials (2022). DOI: 10.1002/adfm.202110716