Artificial muscles that mimic the motions of natural muscles have the potential to revolutionize healthcare. Considered soft robots, these devices, which are actuated thermally, electrically, pneumatically, or by other means, can be implanted in the human body to compensate for diseased or defective muscles. Their widespread clinical use could address the massive need for organ donors—particularly heart transplants—which are in short supply globally.
For example, soft robotic sleeves that wrap around the heart have been developed to assist the ventricles in pumping blood throughout the body. Although these artificial muscles are still in the early stages of research, they have the potential to treat heart failure in patients with cardiovascular disease, significantly enhancing their quality of life—at least while awaiting a natural heart transplant.
Recently, the HybridHeart project, consisting of a consortium of researchers from several European research institutions, received over 10 million euros in funding to develop a unique soft robotic heart, which is anticipated to be ready for clinical trials within the next seven years. This hybrid heart, consisting of a soft shell with artificial muscles and sensors as well as an inner lining composed of the patient’s own cells, would be a permanent implant that completely eliminates the need for an eventual heart transplant.
Heart failure isn’t the only condition that could be remedied by artificial muscles.
Recently, researchers at the University of Grenoble Alpes, France, developed an artificial muscle that stimulates insulin production in pancreatic cells when an external magnetic field is applied. This device could one day be used to treat diabetes.
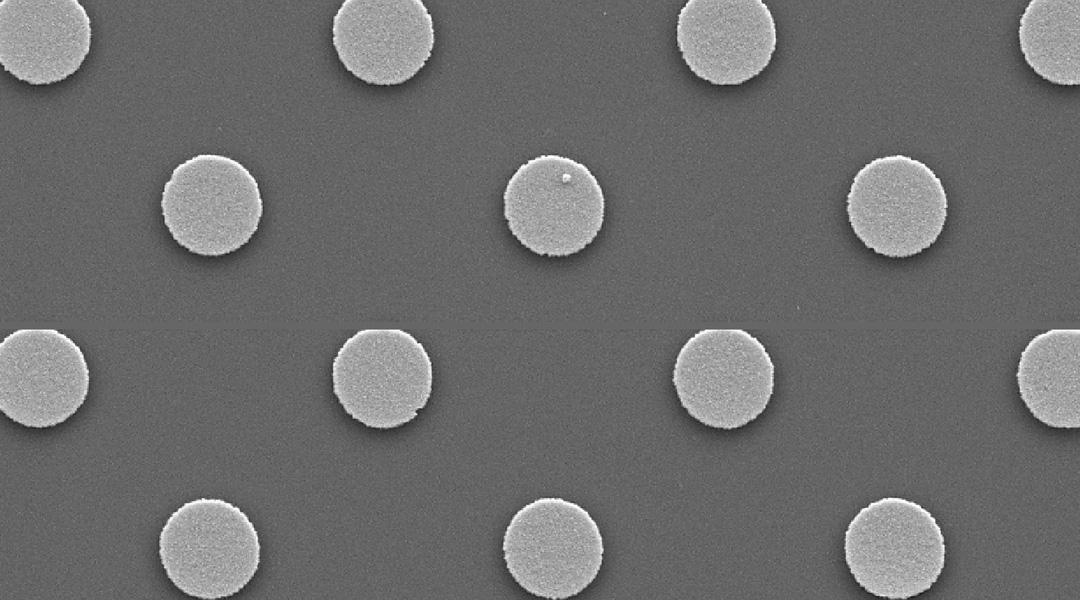
The 1.4-centimeter-diameter polydimethylsiloxane (PDMS) membrane contains around 17 million nickel–iron magnetic particles in the form of microsized disks, which are invisible to the naked eye. A regular array of these microdisks was embedded into the PDMS membrane using deep UV photolithography and evaporation techniques. This fabrication approach allows the membrane to undergo large mechanical deformations because it does not require an additional gold layer for electrodeposition, which would decrease the membrane’s flexibility, a major disadvantage of previously fabricated membranes.
“With the advantage of adjustable elasticity, the range of elasticity of these membranes is similar to that of certain living tissues, such as muscle, skin, bladder and cornea,” Hélène Joisten, one of the researchers who developed the membrane, explained. “This makes them suitable for interacting with cells or biological tissues and for mimicking certain biologically active behaviors of the cellular microenvironment through their magnetic actuation.”
The study of how mechanical forces affect cellular biological processes is known as mechanobiology and has been applied in cancer treatment. In vitro experiments have shown that dispersed magnetic nanoparticles and micro- or nanosized disks can kill cancer cells through magnetomechanical actuation. When introduced to cancer cells, these particles or disks either bind to the cell surface or are internalized by the cell, inducing apoptosis by vibrating in the presence of an applied magnetic field.
A magnetomechanical approach was also applied by Joisten and her colleagues. “The advantage of magnetism is the ability to actuate the membrane remotely and in a tunable way (i.e., the amplitude of vibration and frequency) by externally applied magnetic field,” she stated.
“The membrane remains flat at rest in zero magnetic field and bends when deformed by the applied magnetic field. The magnetic forces on each particle are in the piconewton range, comparable to certain forces acting at the level of biological cells in living tissues.”
By culturing pancreatic cells directly on the membrane, insulin production was significantly increased under mechanical vibrations of the membrane, induced by a rotating magnetic field.
Another important advantage of the magnetic-microdisk-embedded membrane is that it works extracellularly to stimulate insulin production, that is, from outside the pancreatic cells. Although internalization of magnetic particles is beneficial for destroying cancer cells, it may cause adverse effects when it comes to stimulating cellular functions.
“Dispersed magnetic particles can be invasive, being internalized by cells or dispersed in the body. By embedding the particles in the polymer film, the system becomes less invasive at the microscopic particle level,” Joisten explained.
“This exploratory study represents the first step towards demonstrating the importance of mechanical stress at the cell level in relation to insulin production,” she added.
Reference: Hélène Joisten et al., Microstructured Magnetoelastic Membrane for Magnetic Bioactuators and Soft Artificial Muscles Applications. Advanced Intelligent Systems (2023). DOI: 10.1002/aisy.202300022
Feature Image: SEM image of embedded magnetic microparticles before PDMS spin coating