The merging of electronics with biology—bioelectronics—is a rapidly growing field of interest in materials science and biomedical engineering. So far, research has mainly focused on healthcare applications, in which minimally invasive electronic devices that integrate with the human body in order to perform a specific function—monitoring a physiological process, diagnosing a disease, or repairing damaged tissue—have led to new ways of addressing a range of medical issues.
For example, biomedical implants—some of which can even be delivered by ingestion—that are designed to deteriorate after their use has been fulfilled is one fascinating technology to emerge from bioelectronics research. Not only does this ensure that the device remains in the body only for its period of clinical utility but renders risky surgical procedures to remove it unnecessary.
The possibility of extending bioelectronics to other organisms—plants—is also beginning to be explored. Engineering plants with a higher tolerance to environmental stress caused by extreme weather conditions such as drought is becoming especially important as the effects of the changing climate manifest.
Dr. Eleni Stavrinidou, who leads the Electronic Plants (e-Plants) subgroup in the Laboratory of Organic Electronics at Linköping University, Sweden, is one researcher pioneering this field. In a recent paper in Small, she presents a device designed to deliver the phytohormone abscisic acid (ABA) into an intact tobacco plant—the first example of an implantable bioelectronic device in plants.
ABA, a large biomolecule, is one of the main hormones involved in regulating plant response to stress, inducing closure of the stomata in drought conditions in order to restrict water loss. Stomata also play a key role in photosynthesis by regulating gas exchange.
The organic electronic ion pump (c-OEIP) developed by her team is minimally invasive for the plant and delivers ABA electronically into the leaf apoplast, inducing closure of the stomata. Conventional OEIP devices have large dimensions and use planar geometries that hinder implantation into soft tissue, whereas this novel, miniaturized device based on glass capillary fibers has both considerably reduced device dimensions and a simplified fabrication process.
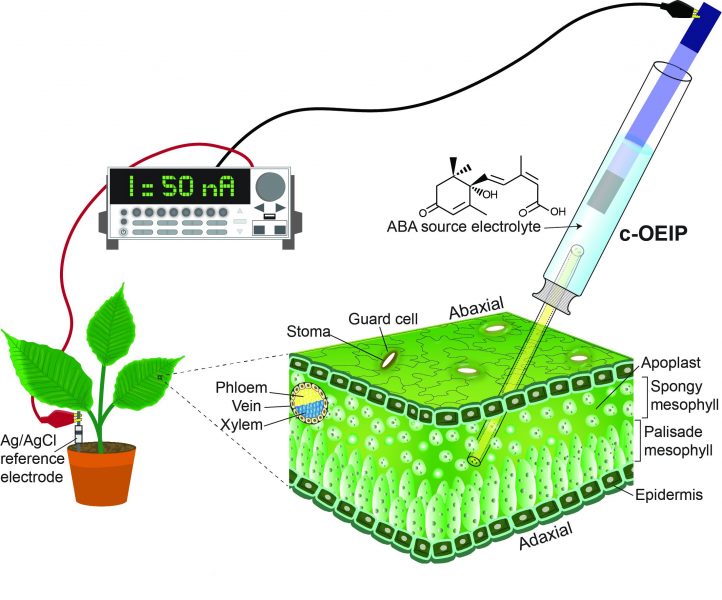
“This work paves the way for establishing bioelectronic devices as versatile tools for biology and the use of bioelectronic devices not only in biomedicine but also in agriculture and forestry,” states Dr. Stavrinidou.
Achieving controlled delivery of even larger signaling molecules, with more dynamic capacity and single-cell resolution, is the next challenge she anticipates in this research.

















