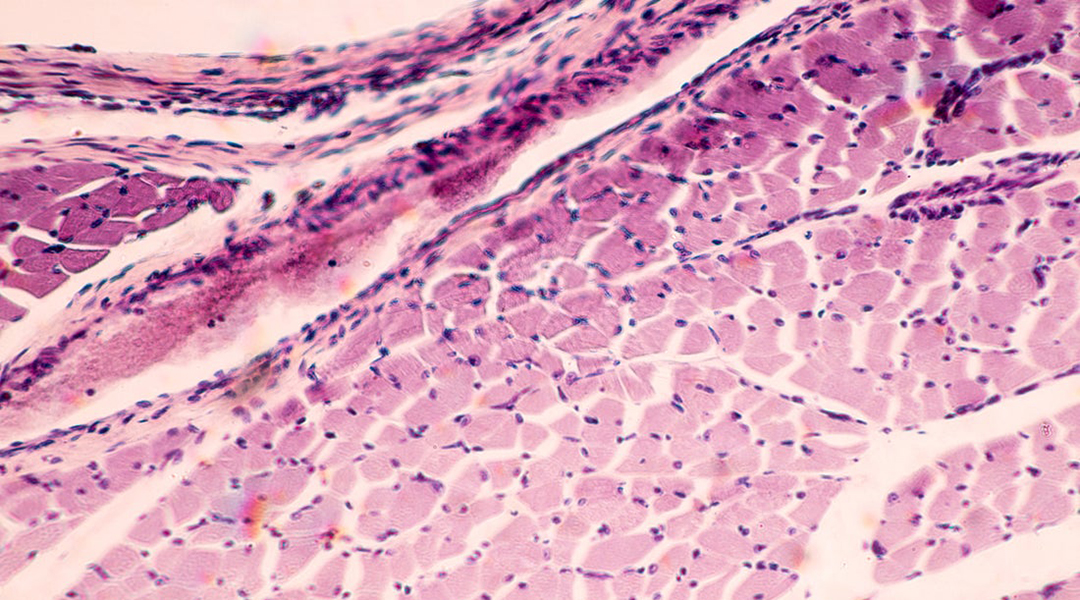
Skeletal muscle under microscope. Image credit: Shutterstock
Researchers from the Center for Nanomedicine within the Institute for Basic Science (IBS), Yonsei University, and the Massachusetts Institute of Technology (MIT) have demonstrated the ability to successfully regrow muscle tissue in mouse models from transplanted cells.
Skeletal muscles do have the ability to regrow on their own, but large volume muscle loss, which can occur following serious injury or treatments such as tumor removal, requires interventional support. These generally include surgical grafts followed by physiotherapy, and while patients can experience some degree of recovery, these surgical procedures often lead to reduced muscle function and some patients even experience complete graft failure.
“These procedures require a secondary surgical site, which can result in donor site morbidity, and may fail to provide effective reconstruction or functional re‐innervation of the injured muscle tissue,” wrote the authors. “These shortcomings often result in chronic functional deficits, which may lead to long‐term disability, weakness, or compromised quality of life.”
To improve long-term recovery, research in recent years has explored inducing natural regeneration of skeletal muscle through transplanted stem cells. While researchers are hopeful that this is a path forward in regenerative medicine, limitations have hindered the translation of this treatment into a clinical setting.
For one, growing enough cells at the site of injury — where millions to billions of mature cells may be needed to provide therapeutic benefits — is still challenging, on top of ensuring that the transplanted cells properly differentiate into muscle tissues with desirable structures. This is generally achieved using synthetic or natural scaffolds that are used to control the cells’ microenvironment and promote growth. However, there are still issues related to how well these can support new tissue development and how well they can integrate within host tissue without being rejected.
In their study published in Advanced Materials, the collaborative team overcame these challenges by implementing a new tissue regeneration strategy that employs direct cell reprogramming in combination with a new hybrid scaffold made from a porous material and a decellularized muscle extracellular matrix hydrogel.
Direct cell reprogramming is key to creating the new tissue, and is an attractive approach for creating new tissue where there is a shortage of proliferating cells for repair. This strategy allows scientists to “re-engineer” cells that are already abundant in the body, changing their function in order to provide enough new cells to restore normal tissue in a damaged area. There is also the added benefit that a patient’s own cells can be used, increasing safety and rate of success.
In the current paper, fibroblasts — cells commonly found in connective tissues and involved in natural wound healing — were converted into a type of progenitor cell called induced myogenic progenitor cells (iMPCs). Progenitor cells can be thought of as descendents of stem cells, which when exposed to specific biomarkers, such as transcription factors, can differentiate to create specialized cells, such as new muscle cells.
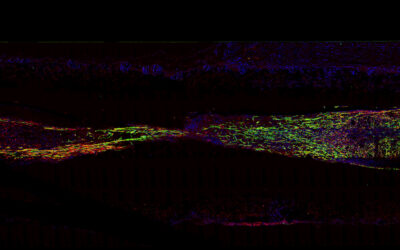
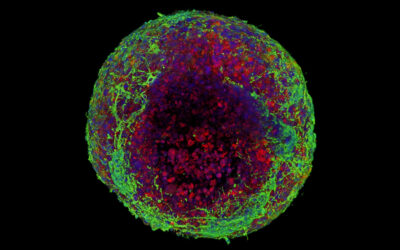
The resultant bioengineered muscle fiber constructs showed stiffness similar to that of muscle tissues and exhibited enhanced muscle differentiation and elongated muscle alignment in vitro. Implantation of bioengineered muscle constructs in mouse models not only promoted muscle regeneration with increased innervation and angiogenesis but also facilitated the functional recovery of damaged muscles.
“The hybrid muscle construct might have guided the responses of exogenously added reprogrammed muscle cells and infiltrating host cell populations to enhance functional muscle regeneration by orchestrating differentiation, paracrine effect, and constructive tissue remodelling,” wrote the team.
“Further studies are required to elucidate the mechanisms of muscle regeneration by our hybrid constructs and to empower the clinical translation of cell-instructive delivery platforms,” concluded the study’s lead, Professor Cho Seung-Woo.
Reference: Yoonhee Jin, et al., Functional Skeletal Muscle Regeneration with Thermally Drawn Porous Fibers and Reprogrammed Muscle Progenitors for Volumetric Muscle Injury, Advanced Materials (2021). DOI: 10.1002/adma.202007946