Researchers from CNRS, Paris, France, recently reported the first comparative study on production, engineering and purification of extracellular vesicles (EVs) as a new generation of personalized drug delivery vectors.
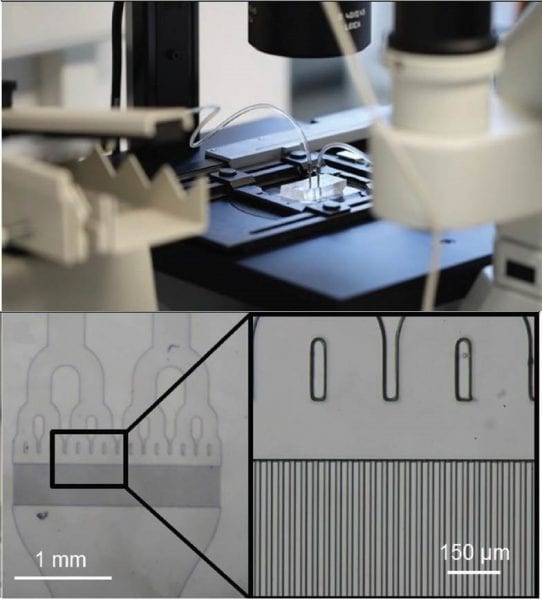
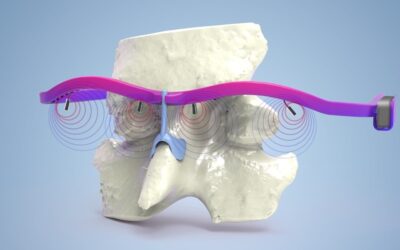
Mechanical microfluidic method for EV production mimicking the natural release process of EVs in blood capillaries.
EVs which are naturally released by cells or in response to stress can act as an intercellular communication pathway. Due to this, extracellular vesicle research has gained increased attention, particularly when combined with therapeutic and/or imaging agents. Despite the great potential of this approach, only few studies have addressed and compared methods to design, purify and characterize EVs for exogenous imaging and therapeutic agents mainly because of challenges with production, purification and characterization processes.
The reported work sheds light on these challenges by comparing three experimental set-ups to produce EVs involving a model drug (photosensitizer) and imaging agent (magnetic nanoparticles) from cells loaded beforehand with these components. For this, the researchers developed a microfluidic method for fast triggering of EV release by endothelial cells which compares the mechanical stress to the starvation stress and to the spontaneous release in complete medium. They also compared two isolation protocols with ultracentrifugation and magnetic sorting to purify the obtained EVs. This demonstrated that EV production by starvation together with purification by ultracentrifugation can be considered a reasonable trade-off between loading, yield and purity for biogeneration of theranostic EVs
This study offers the benchmark for a rational choice of production and purification methods to create theranostic EVs which represents a step forward in the long road towards clinical translation.