Lithium-sulfur (Li-S) batteries are considered to be one of the most competitive candidates for “post-lithium” technology due to their advantageous cost and specific energy. Nevertheless, the practical application of Li-S batteries is still hindered by unavoidable capacity decay due to continuous dissolution/shuttle of soluble polysulfide species during repeated discharge-charge processes, as well as limited utilization and poor electrochemical reversibility of the active material, which stems from the insulating property of sulfur. While much work has focused on designing a proper carbon structure to achieve strong carbon-pore confinement of sulfur and its soluble discharge intermediates, the conduction problems of sulfur and its discharge product (Li2S) have rarely been studied.
Lithium-sulfur (Li-S) batteries are considered to be one of the most competitive candidates for “post-lithium” technology due to their advantageous cost and specific energy. Nevertheless, the practical application of Li-S batteries is still hindered by unavoidable capacity decay due to continuous dissolution/shuttle of soluble polysulfide species during repeated discharge-charge processes, as well as limited utilization and poor electrochemical reversibility of the active material, which stems from the insulating property of sulfur. While much work has focused on designing a proper carbon structure to achieve strong carbon-pore confinement of sulfur and its soluble discharge intermediates, the conduction problems of sulfur and its discharge product (Li2S) have rarely been studied.
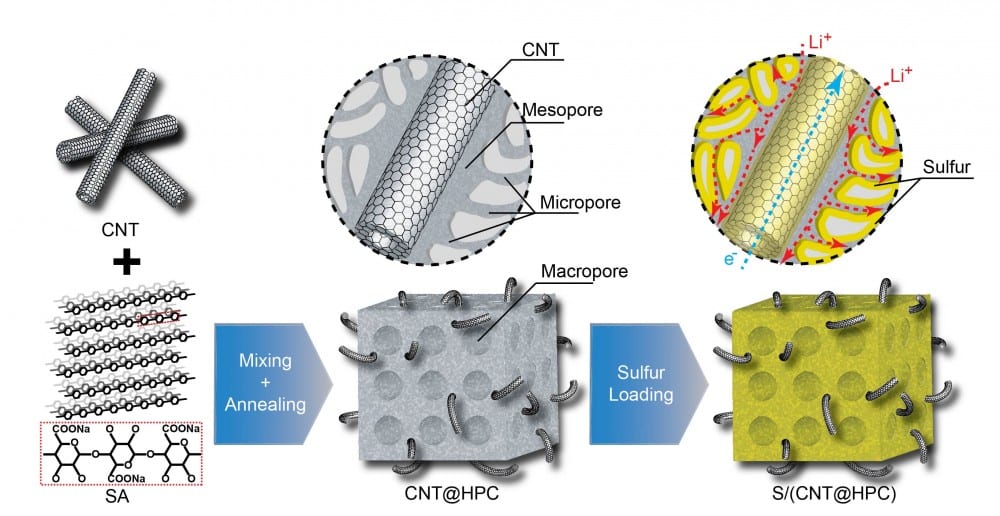
To address the above problems, Huai-Ping Cong’s group from Hefei University of Technology and Shu-Hong Yu’s group from University of Science and Technology of China proposed a facile, scaled-up synthesis strategy to improve the performance of the sulfur cathode by embedding multi-walled carbon nanotubes (CNTs) inside a biomass-derived hierarchically porous carbon (HPC) for the subsequent sulfur loading to yield a S/(CNT@HPC) cathode. In their report in ChemNanoMat, they show that the porous carbon provides sufficient confinement to trap soluble polysulfides within the cathode, and that the embedded CNTs form highly efficient and robust conductive pathway for electrons, enabling significantly improved cathode conductivity and also contributing to the structural integrity of the cathode.
To overcome the energy barrier in the delithiation process of Li2S, they further employed a potentiostatic process at the end of charging (at 2.8 V), and found the reversibility of the cathode delithiation reaction is significantly improved, bringing high capacity retention and decreased polarization upon cycling. This strategy not only provides a practical and facile way to fabricate a sulfur composite cathode material with favorable electrochemical properties, but also gives insights on how to optimize the cathode design for Li-S batteries.
This article is part of a special issue on Nanomaterials for Energy Conversion and Storage in ChemNanoMat ((link to: www.chemnanomat.org)). Click here ((link to: http://onlinelibrary.wiley.com/doi/10.1002/cnma.v2.7/issuetoc)) to see the other articles in the issue.