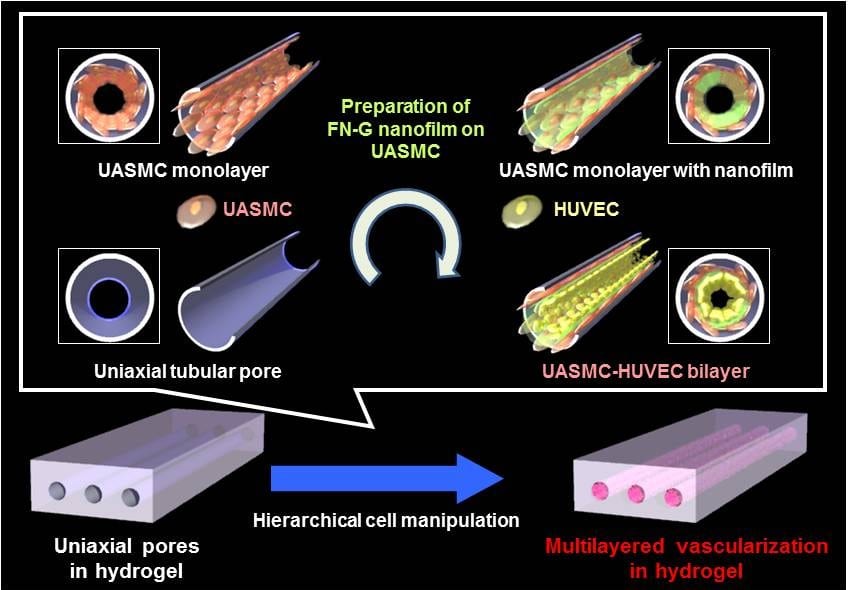
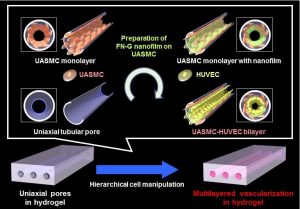
Blood capillaries play a critical role in biomedical research, from the evaluation of drug permeabilities to the study of cancer metastasis. In general, in vivo animal studies are employed in this field, which invariably leads to some uncertainty when the results are applied to humans. For this reason, there is a great need for the in vitro reconstruction of blood capillaries.
A team from Osaka University has recently made an important advance by fabricating multilayered blood capillary analogs inside biodegradable hydrogels. They achieved this by forming the gel in the presence of silica capillary tubes, which were subsequently extracted, leaving behind microchannels in the gel matrix. This method is quite simple and extremely versatile, in that control over microchannel diameter, length, and orientation is afforded. To transform the naked microchannels into effective blood capillary analogs, they coated the walls of the microchannels with layers of endothelial and smooth muscle cells using a technique recently developed in their group (see figure). As the number of layers of smooth muscle cells in real blood capillaries varies depending on the tissue type and anatomical location, the fact that control over this parameter is achieved with this technique is a major advantage, and is crucial for effective use in drug permeability studies.
 To viably use these capillaries for in vitro bioassays, their permeation and barrier properties must be comparable to those of native blood capillaries. The researchers were able to demonstrate that, whereas the gel network itself is highly permeable, the bilayered microchannels exhibit a strong barrier effect, similar to their native counterparts. In addition to this, owing to the biodegradable nature of the hydrogel support used, the multilayered capillaries can easily be collected through simple chemical reduction and degradation of the gel.
To viably use these capillaries for in vitro bioassays, their permeation and barrier properties must be comparable to those of native blood capillaries. The researchers were able to demonstrate that, whereas the gel network itself is highly permeable, the bilayered microchannels exhibit a strong barrier effect, similar to their native counterparts. In addition to this, owing to the biodegradable nature of the hydrogel support used, the multilayered capillaries can easily be collected through simple chemical reduction and degradation of the gel.
This new platform will likely become an important biomedical research tool, not only for studying the permeation of drugs and cancer cells, and providing valuable information not accessible from in vivo bioassays, but also for artificial blood capillary implantation.