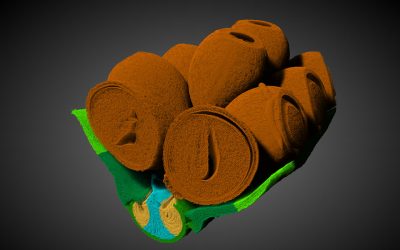
A wide range of scientific fields are currently benefiting from the use of porous materials, making them a hot research topic. Their primary use is to use their pores to grab and store particles, often scooping them out from a solution.
Yet, despite finding applications in energy storage and conversion, cleaning up environmental hazards, in chemical production, biomedicine, and agriculture, these materials come with a range of pore sizes — from macroscopic bulk materials with millimetre-scale pores, such as sponges or foams to tiny particles with sub-nanometre scale pores, such as metal-organic frameworks and porous graphene.
Materials with these different pore sizes possess different qualities, strengths, and weaknesses. Bulk materials with relatively massive pores have good mass transfer rates and stability but demonstrate low utilization of the inner space. Tiny particles with smaller pores, conversely, have high surface area meaning lots of sites to bind particles but can be brittle and tough to use.
These differences have driven the desire to create a single so-called “hierarchical” material with mixed pore sizes that can have the strengths of both macroscopic bulk materials and tiny particles. Yet progress in this direction has been limited and fabrication of a multi-pore-sized remains challenging and expensive.
A hierarchical porous material inspired by the blood
In a new paper published in the journal Advanced Science authored by Lin Zhang and Yingchun Fu, researchers at the Intelligent Bioindustrial Equipment Innovation Team (IBE), led by Professor Yibin Ying at Zhejiang University, report a new way of fabricating a multi-pore-sized material is suggested that takes blood-curdling inspiration from the human body.
“Nature can provide a rich source of inspiration for materials design and utilization. The inspiration for this material is an interesting story,” said Zhang. “Blood coagulation is a common but delicate physiological behavior in living systems. In its final stage, a protein called fibrinogen polymerizes into fibrin networks to form a clot with the adhesion of lots of blood cells, thereby sealing and facilitating the healing of wounds with diameters from micrometers to even centimeters.”
Zhang explained that these wounds are analogous to the large pores of the macroscopic bulk materials while the blood cells are similar to their tiny particles. Thus, the healing process inspired them to use fibrin as the sub-framework to integrate the macroscopic bulk materials with their large pores and tiny particles with smaller pores thus bridging the vast gap in the scale of the pore sizes between the two.
The resultant material has three tiers, which see macroscopic bulk materials acting as the primary structure for robust support while fibrin serves as the second-level bridging framework for the weaving of larger pored and smaller pored materials. Finally, metal–organic frameworks make up the third-level structure, acting as the functional elements with large specific surface areas and an abundance of active sites for scooping up molecules. The result is a material that scoops up targets in solution with high speed and capacity.
The researcher added that in the team’s previous works, fibrin was demonstrated to be highly adhesive to various materials, including inorganic nanomaterials and organic biomaterials, hence the reason they chose to use it in this material.
Bumps in the road
Constructing a fibrin-based, three-level hierarchical porous structure was an attractive but challenging task for the team, and one that delivered a few surprises despite their previous experience.
“We tried several methods and conditions to achieve this advanced structure of the material, Fu explained. “In the beginning, we tried to fabricate the materials by one-step addition of reactants like fibrinogen, thrombin, and tiny particles to the macroscopic bulk materials.”
Fu went on to say that when the team did this, the fibrin presented as a dense film that coated the frameworks of the macroscopic bulk materials and the surface of the tiny particles, leaving the massive internal space of the materials unused.
The team found that the preformed fibrin networks could capture and attract the tiny particles and these could, in turn, act as the anchors for the subsequent formation of fibrin nanofibers. This allowed for more and more tiny particles to be added to the bulk material stably.
“Fortunately, when we changed to the separated addition of the reactants for fibrin and tiny particles, the hierarchical porous structure was obtained,” said Fu. “The structure is unique and exciting, which surprised us.”
Porous materials are better in all sizes
A new form of hierarchical porous macroscopic material is needed, said Zhang, explaining that current platforms contain two kinds of pores fabricated either by processing pre-formed tiny particles into materials or by modifying particles on the frameworks of the materials.
“The products obtained by the former strategies suffer from ill-defined pore structure and the aggregation of the tiny particles, which could cause limited diffusion and mechanical stability for applications,” said Zhang.
She added that on the other hand, the latter strategy results in massive internal space of the large pores of the material being left unexplored with few tiny particles loaded resulting in reduced function.
“In our opinion, the vast gap between the cross-scale pores of macroscopic bulk materials and tiny particles is one of the most significant obstacles that hinder the combination of these materials to fabricate the hierarchical porous macroscopic materials,” she said.
The blood coagulation-inspired process developed by the team avoids these problems by offering a dynamic bridging strategy with fibrin, with an appropriate pore structure to load the smaller pored tiny particles into.
Adding to the usefulness of this strategy, the team said that it makes the fabrication of the hierarchical material rapid, controllable, and universal, with just two steps. The resultant product is stable with a high load capacity possessing a novel three-level hierarchical porous structure.
The team will now work on the modification of fibrin to achieve fibrin-based materials with elasticity to fabricate flexible materials for wearable devices. In addition to this, they hope to make the creation method more affordable allowing it to be widely adopted and scaled-up.
Reference: Yingchun Fu, et al., Blood-Coagulation-Inspired Dynamic Bridging Strategy for the Fabrication of Multiscale-Assembled Hierarchical Porous Material, Advanced Science, (2022). DOI: 10.1002/advs.202204702