 The transplantation of engineered functional matrices is rapidly gaining attention as a way of regenerating organotypic tissues for replacement, renewal or reinforcement purposes.
The transplantation of engineered functional matrices is rapidly gaining attention as a way of regenerating organotypic tissues for replacement, renewal or reinforcement purposes.
Cells can be seeded within three-dimensional (3D) spaces to proliferate into functioning tissues in a lab. The implantation of these matrices on the damaged tissue can aid in the healing and recovery process through the secretion of bioactive factors or by integration with the natural cell system.
As a result, the synthetic engineering of tissue engineering matrices was focused on specific features of the extracellular microenvironment, such as mechanical properties, chemical nature and topology. Synthetic polymers or processing methods could mimic the fibrous structure and topology, scaffolds with similar biochemical composition can be designed, or scaffolds can be designed with matching mechanical properties.
For example, various biodegradable polymers, such as poly(ϵ-caprolactone) (PCL) have been electrospun to form non-woven scaffolds for tissue engineering. However, the mismatch of the mechanical properties with that of native tissues and the lack of cell-interactive bioactive molecules hinder their applications. Natural biomolecules which can provide cues for tissue assembly lack the viscoelastic properties leading to the formation of unstable structures when fabricated on its own. Therefore, typical fabrication processes involve the mixing of both synthetic and natural molecules.
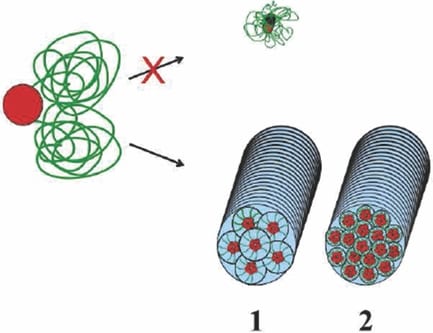
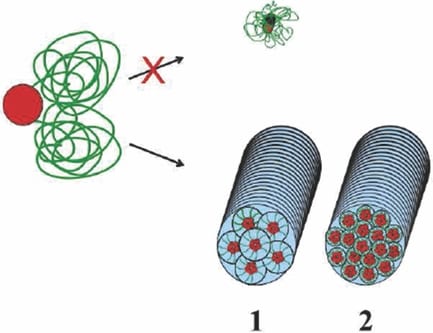
Recently, Professor George Fytas from the University of Crete and F.O.R.T.H and Dr Markus Klapper from the Max Planck Institute for Polymer Research reported an attempt to mimic nature’s concepts in forming synthetic hydrogel fibers via a bottom-up self-assembly process. This was achieved by carefully adjusting the molecular interactions of different amphiphiles. The purely synthetic fibers made from hexaphenylbenzene-poly(ethylene glycol) (HPB-PEG) amphiphiles form bundles of hydrogel fibers in very dilute aqueous solution, which was controlled by different molecular interactions. By controlling the length of the PEG chains and the substitution pattern of the precursor molecules, the amount of water in the hydrogel fiber can be controlled. The direct impact of such a system would be the adjustment of the mechanical properties of the fibers by controlling the composition of the amphiphile. The synthetic approach also offers possibilities in the conjugation of bioactive ligands to the molecules.
This paper uncovers fundamental knowledge required for fiber formation. It will be interesting to understand and further develop on these concepts by applying the knowledge to aspects of scaling-up such a process and to use it to eventually develop a truly synthetic fibrous matrix resembling an extracellular matrix and yet offering the flexibility of being able to fine-tune the chemical and mechanical properties by the appendage of bioactive as well as functional groups.