Image credit: Efe Alpay
Biological actuation systems that pump bodily fluids or enable locomotion are self-assembled from microscale units, otherwise known as muscle cells. Muscle cells transform chemical energy into mechanical work through an actomyosin network. This intracellular machinery is dynamically self-assembled from nanoscale building blocks and powered by biological motors called myosin proteins.
The hierarchical organization makes biological actuation systems highly scalable, robust against mechanical injury, and adaptive to the requirements of different uses. Robotic systems may possess similar properties if we can discover rapid and efficient powering and control schemes for the bottom-up assembly of actuated parts according to the desired blueprint.
Programmable self-assembly methods are expected to play a key role in the development of advanced medical devices because these methods can significantly enhance accessibility, reduce the invasiveness associated with current devices, and provide flexibility to tailor the final configuration.
A team of engineers led by myself from the Institute of Mechanical Engineering at École Polytechnique Fédérale de Lausanne (EPFL) has introduced a collection of materials and methods that enable remotely controlled assembly of microscopic robots from nanoscale machines. Our findings were recently published in Advanced Intelligent Systems.
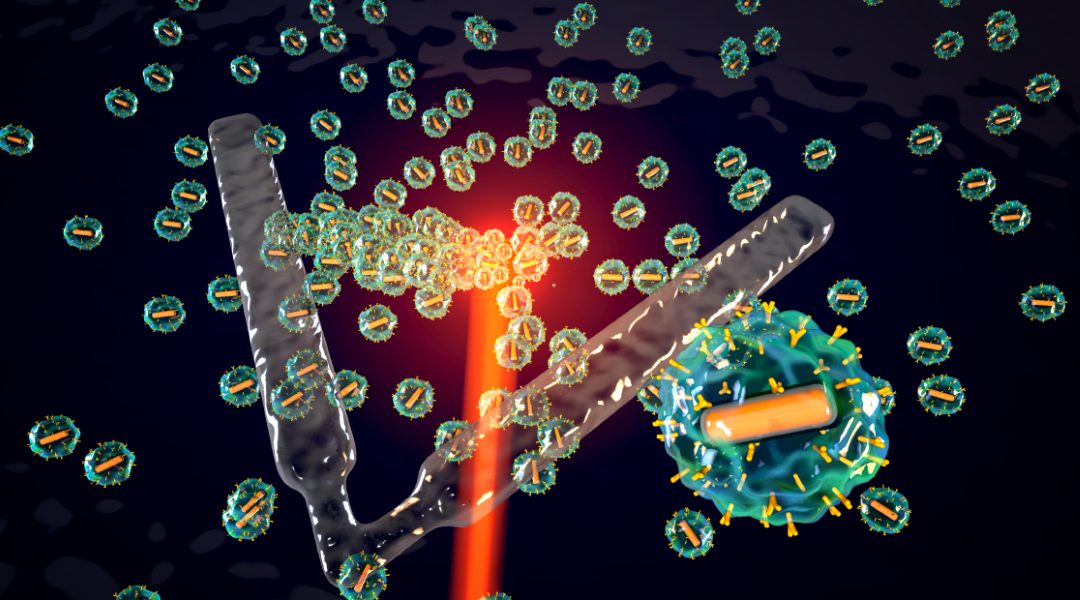
We synthesized nanoparticles serving as untethered machines that are capable of transducing wireless energy of various forms into mechanical work. Each particle has a magnetized gold nanorod at its core, which serves as a photonic heater that converts optical energy into thermal energy, and as a motor that generates motion when driven by magnetic fields. The metal core is encapsulated inside a thermoresponsive gel that rapidly converts generated heat into deformation and linear actuation. Groups of particles can be collected at a desired location using a laser beam or clustered into dynamic configurations using time-varying magnetic fields. While near infrared illumination generates thermocapillary flows that transport particles to hot regions, application of magnetic torque promotes the formation of chains through dipole-dipole and hydrodynamic interactions.
“The proper assembly of the microrobot requires robust attachment of the building blocks with each other and other mechanisms,” said Raquel Parreira, my colleague and the lead author of the study. “We addressed this issue by functionalizing the surfaces of the particles with amine groups and incorporating platinum into the metal core. Platinum catalyzes covalent bonding of amine groups with the aid of localized heating, thereby completing the robot morphogenesis.”
Once the assembly process is completed, the same optical and magnetic signals are used to power the robot. Actuators with many degrees of freedom can be created for controlled locomotion in fluids and dexterous manipulation of target objects. The robots can be upgraded or repaired by simply re-initiating the assembly process in the presence of the building blocks. The chemical and mechanical compatibility of the chosen materials with living cells makes the presented technology promising for biomedical applications. The microrobots will be assembled in situ at the target location from injected nanoparticles.
In addition, recapitulating muscle architecture in fully synthetic biomimetic micromechanical systems will allow systematic investigation of biological actuation.
Reference: Raquel Parreira, et al., ‘Remotely Controlled Colloidal Assembly of Soft Microrobotic Artificial Muscle, Advanced Intelligent Systems‘ (2020). DOI: 10.1002/aisy.202000062