North Carolina State University researchers have shown that the “bulkiness” of molecules commonly used in the creation of gold nanoparticles actually dictates the size of the nanoparticles – with larger so-called ligands resulting in smaller nanoparticles. The research team also found that each type of ligand produces nanoparticles in a particular array of discrete sizes.
“This work advances our understanding of nanoparticle formation, and gives us a new tool for controlling the size and characteristics of gold nanoparticles,” says Dr. Joseph Tracy, an assistant professor of materials science and engineering at NC State and co-author of a paper describing the research. Gold nanoparticles are used in industrial chemical processes, as well as medical and electronics applications.
When creating gold nanoparticles, scientists often use organic molecules called ligands to facilitate the process. The ligands effectively bring gold atoms together in a solution to create the nanoparticles. In the process, ligands essentially line up side by side and surround the nanoparticles in all three dimensions.
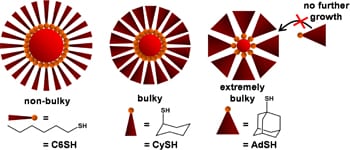
The researchers wanted to see whether the bulkiness of the ligands affected nanoparticle size, and opted to assess three types of thiol ligands – a family of ligands commonly used to synthesize gold nanoparticles. Specifically, the molecules bound to the gold nanoparticles are linear hexanethiolate (-SC6), cyclohexanethiolate (-SCy) and 1-adamantanethiolate (-SAd). Each of these ligands has a bulkier configuration than the last.
For example, picture each ligand as a slice of pie, with a gold atom attached to the pointed end. -SC6 looks like a very narrow slice of pie. -SCy is slightly larger, and -SAd is the largest of the three – with the “crust” end of the pie wedge far wider than the pointed end.
The researchers found that the bulkiness of the ligands determined the size of the nanoparticles. Because fewer -SAd and -SCy ligands can line up next to each other in three dimensions, fewer gold atoms are brought together in the core. Therefore, the nanoparticles are smaller. -SC6, the least bulky of the thiolates, can create the largest nanoparticles.
“While we’ve shown that this is an effective means of controlling size in gold nanoparticles, we think it may have implications for other materials as well,” says Peter Krommenhoek, a Ph.D. student at NC State and lead author of the paper. “That’s something we’re exploring.”
But the researchers made another interesting finding as well.
When particularly small nanoparticles form, they tend to form at very specific sizes, called discrete sizes. For instance, some types of nanoparticles may consist of 25 or 28 atoms – but never 26 or 27 atoms.
In this study, the researchers found that the bulkiness of the ligands also changed the discrete sizes of the nanoparticles. “This is interesting, in part, because each discrete size represents a different number of gold atoms and ligands,” Tracy says, “which could influence the nanoparticle’s chemical behavior. That question has yet to be addressed.”
The paper, “Bulky Adamantanethiolate and Cyclohexanethiolate Ligands Favor Smaller Gold Nanoparticles with Altered Discrete Sizes,” was published online June 15 in ACS Nano. The paper was co-authored by Dr. Junwei Wang, a former postdoctoral research associate at NC State; Dr. Nathaniel Hentz, of the Golden LEAF Biomanufacturing Training and Education Center at NC State; Krystian Kozek, a former undergraduate at NC State; Dr. Aaron Johnston-Peck, a postdoctoral researcher at Brookhaven National Laboratory; and Dr. Gregory Kalyuzhny of San Diego State University.
The research was supported by the National Science Foundation and the U.S. Department of Education.