In the search for effective anticancer therapy, growing attention is being paid to photodynamic therapy (PDT), but what makes this treatment option so special?
Its appeal lies in the possibility of using non-toxic compounds that acquire cytotoxic properties only upon exposure to light of specific wavelength, an advantage over classical chemotherapy.
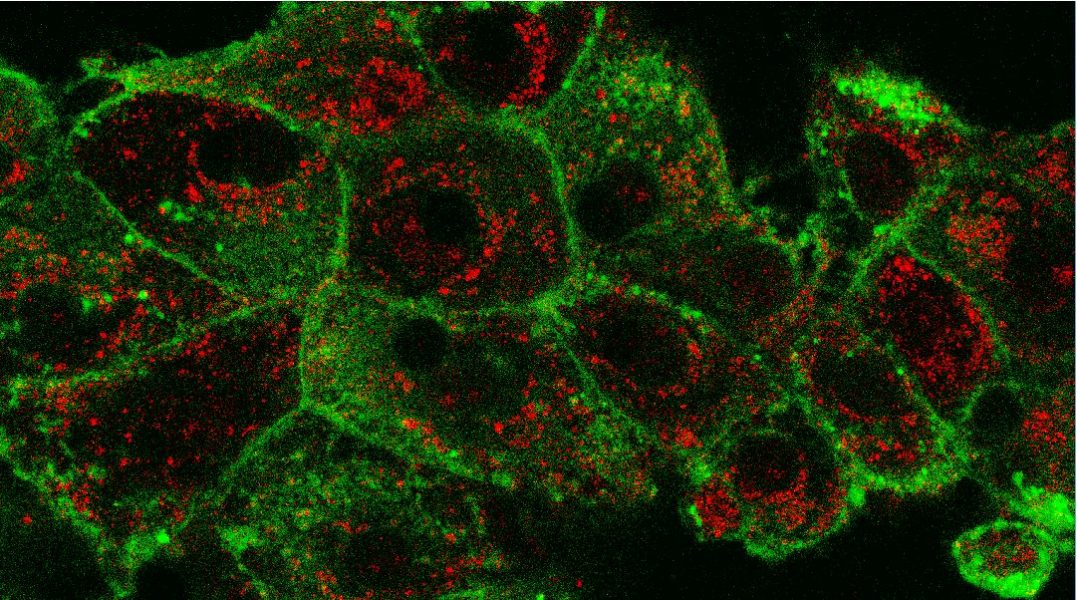
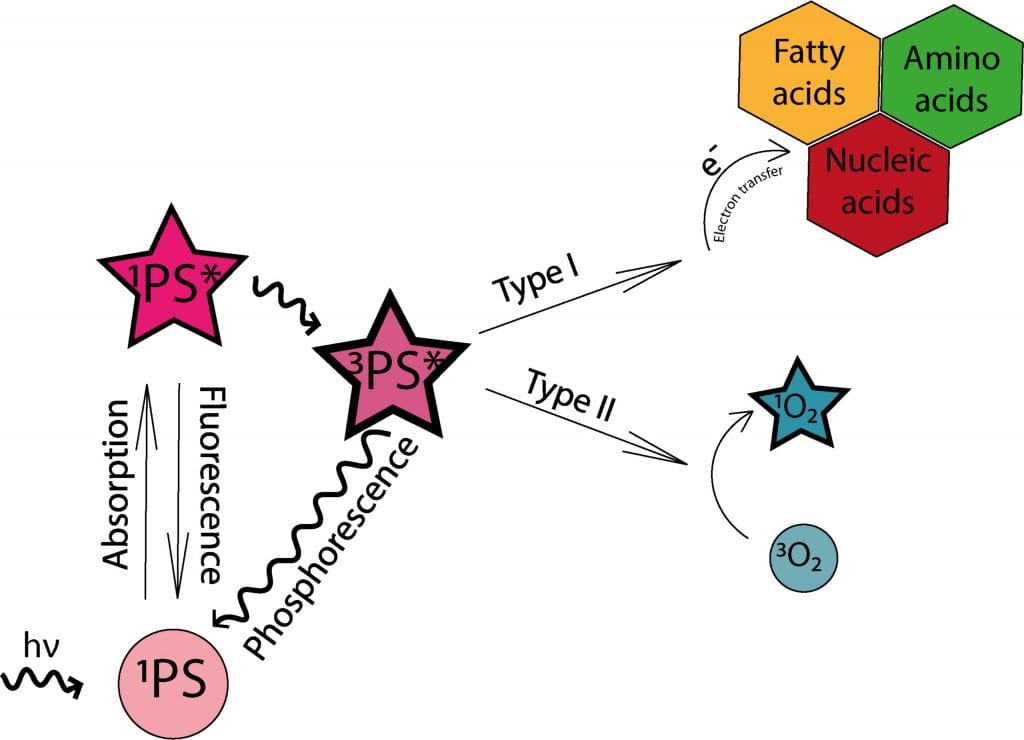
In PDT, light-sensitive therapeutics called photosensitizers are injected into the bloodstream or directly into the tumor. These compounds contain chromophore molecules that transfer their energy to cellular oxygen upon irradiation, resulting in the formation of singlet oxygen and other reactive oxygen species (ROS) that may cause significant damage to the cell structures or tumor blood vessels and trigger the anticancer activity of the immune system.
However, PDT is not without limitations, including hampered cellular uptake and poor biodistribution of photosensitizers. The latter may additionally cause mild but long-lasting side effects such as sensitivity to light—leading to burns, swelling, pain, and scarring in healthy tissues surrounding the tumor. Another obstacle is poor light penetration through tissues, reducing the applicability of PDT to small tumors located on or just underneath the skin.
Currently, researchers are on a quest to expand PDT to different types of cancers and to develop more powerful photosensitizers and methods of targeted delivery. One of the most promising approaches involves the use of carrier systems that can modulate biodistribution as well as pharmacokinetic and pharmacodynamic properties of photosensitizers.
In this regard, the rapidly growing area of nanoscience and nanotechnology may yield nanosized compounds lacking the limitations of the clinically used drug-delivery systems.
It has been shown that nanoparticles have the ability to protect therapeutics from degradation, improve their solubility, prolong blood half-life, and provide targeted delivery and controlled release. This makes nanocarriers a promising alternative to classical PDT, enabling the modification of its critical steps: photosensitizer transport and location, as well as the intensity of photodynamic reaction.
To date, a number of different nanoparticles have been tested for the delivery of photosensitizers, the increase of their cellular concentration, and their phototoxic properties. These include liposomes, dendrimers, gold and silver nanoparticles, and polymersomes, among others.
Some of these nanoparticles are currently being transferred from in vitro to in vivo studies, with polymeric, metallic, and silica nanoparticles giving the greatest hope for the development of modern PDT. These compounds have been shown to enhance tumor accumulation and improve phototoxic effects of the drugs in animal models.
Kindly contributed by the authors.