Raman technique is able to capture changes in early tissue malignancy before any visible morphological changes develop. As technological challenges such as weak signal, long integration time, and strong fluorescence background have largely been overcome, Raman technique is on its way now to be translated into clinical use.
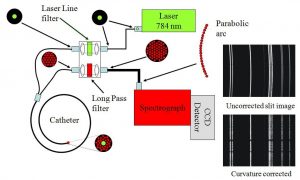 Histopathological examination of biopsied tissue specimens is still the gold standard for cancer diagnosis. Yet, the diagnostic process is time consuming, creates anxiety, and extends procedure time. Diagnosing cancer with satisfactory sensitivities and specificities that spare most benign lesions is still challenging, especially when performed in real-time and in vivo. Raman spectroscopy which developed into a practical tool for rapid in vivo tissue diagnosis is an optical techniques holding a lot of promise as a molecular tool for cancer diagnosis. The reason is that most molecular cancer biomarkers, e.g., proteins, nucleic acids, lipids, and carbohydrates, are responsive to Raman scattering process. In addition, Raman technique is sensitive to structural changes such as nuclear enlargement, a histological signature of carcinoma, and phase transitions. In vivo Raman spectra can be measured by shining laser directly onto suspected cancer lesions without taking a biopsy.
Histopathological examination of biopsied tissue specimens is still the gold standard for cancer diagnosis. Yet, the diagnostic process is time consuming, creates anxiety, and extends procedure time. Diagnosing cancer with satisfactory sensitivities and specificities that spare most benign lesions is still challenging, especially when performed in real-time and in vivo. Raman spectroscopy which developed into a practical tool for rapid in vivo tissue diagnosis is an optical techniques holding a lot of promise as a molecular tool for cancer diagnosis. The reason is that most molecular cancer biomarkers, e.g., proteins, nucleic acids, lipids, and carbohydrates, are responsive to Raman scattering process. In addition, Raman technique is sensitive to structural changes such as nuclear enlargement, a histological signature of carcinoma, and phase transitions. In vivo Raman spectra can be measured by shining laser directly onto suspected cancer lesions without taking a biopsy.
In a review article, researchers from the British Columbia Cancer Agency Research Centre and the University of British Columbia/Vancouver Coastal Health Research Institute (Vancouver, Canada) give an overview on the latest development of real-time in vivo Raman systems for cancer detection. They cover instrumentation, data handling, as well as oncology applications of Raman techniques and discuss optic fiber probes designs for Raman spectroscopy. In addition, spectral data pre-processing, feature extraction, and classification between normal/benign and malignant tissues are surveyed. Applications of Raman techniques for clinical diagnosis for different types of cancers, including skin cancer, lung cancer, stomach cancer, oesophageal cancer, colorectal cancer, cervical cancer, and breast cancer, are summarized.
Haishan Zeng and his co-authors conclude that most notable breakthroughs in Raman instrumentation include design and development of miniature Raman fiberoptic probes and catheters. These innovative probes allow Raman system to be used as an adjunct device to a conventional endoscope. In addition, patented Raman spectrometer designs drastically improve spectral signal-to-noise ratios and reduce required integration time down to seconds. Another important improvement goes ahead with the current boom in data mining and bioinformatics research which greatly facilitates automated analysis of Raman spectra.
The authors assess the clinical outcomes on cancer detection of skin, lung, gastrointestinal tract, cervix, and breast using Raman technique as encouraging.
Results have demonstrated that Raman spectroscopy is effective in reducing the number of false positive biopsies for cancer diagnosis. They hope that larger scale clinical studies will accelerate clinical acceptance of Raman technique. Key research areas are expected to focus on novel designs of Raman fiber optic probes and data handling algorithms to enable fully automated spectra analysis. Raman technique might even become a powerful diagnostic tool during intraoperative procedures.
















