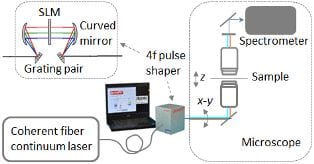
Coherent anti-Stokes Raman scattering microscopy offers noninvasive label-free imaging, high sensitivity, and chemical specificity, which makes it an attractive alternative to histopathology for diagnosis. For clinical translation, some technical barriers still have to be overcome using advanced features and schemes.
Stained histopathology is currently the gold standard for disease diagnosis but remains a subjective practice on processed tissue, taking from hours to days. More quantitative and rapid analysis could be provided by near-infrared Raman microspectroscopy, an attractive alternative which offers a noninvasive assay of the tissue without external staining or labeling. Since pathological changes are often preceded by microscopic chemical alterations, the obtained Raman hyperspectral image and data of the tissue can potentially be used as an early-stage phenotypic set of markers for tissue pathology. However, the weak Raman scattering of common biomolecules necessitates a long image acquisition time of several hours. Coherent anti-Stokes Raman scattering (CARS) microscopy, a nonlinear optical variant of Raman microspectroscopy, holds the promise to shorten this time below minutes. Yet, there are still some restraints that limit the clinical translation of CARS microscopy. Although each of them can be overcome with advanced features, the implementation of one or a small number of these features often introduces more tradeoffs than benefits.
In a review article, Haohua Tu and Stephen A. Boppart from the University of Illinois at Urbana-Champaign (USA) discuss the six most outstanding technical barriers and six advanced features, including interferometry, that can be independently added into a standard but high-performance scheme to overcome these barriers. They also outline a strategy that would integrate multiple advanced features to overcome these barriers simultaneously, effectively reduce tradeoffs, and synergistically optimize CARS microscopy for clinical translation. The operation of the envisioned system incorporates coherent Raman micro-spectroscopy for identifying vibrational biomolecular markers of disease and single-frequency (or hyperspectral) Raman imaging of these specific biomarkers for real-time in vivo diagnostics and monitoring.
By recognizing CARS spectroscopy vs. CARS imaging as the most fundamental tradeoff, the authors suggest that clinical CARS microscopy should be optimized to perform either Raman spectroscopy with a broad spectral coverage, or Raman imaging at one or a few discrete Raman frequencies, but not both. The former could be realized by integrating all of the six advanced features discussed, resulting in a highly sensitive version of spontaneous Raman microscopy that could rapidly identify new Raman biomarkers of medical significance from thin ex vivo tissue sections. The latter would adaptively integrate some of the advanced features, depending on the identified Raman biomarkers and the technical issue of fiber-based miniaturization, to perform in vivo molecular imaging in patients.