Polyolefins are the most important commodity polymers today. They are classified in two broad families, polyethylenes and polypropylenes, that can be subdivided in several different product types. Among those, polyethylenes made with coordination catalysts or free radical polymerization are among the most important polymers in the world.
As shown in their published Feature Article by Vasileios Touloupidis and Andreas Albrecht (Borealis Polyolefine GmbH, Linz, Austria) together with João B.P. Soares (University of Alberta, Edmonton, Canada), the properties of coordination polyethylenes are controlled by changing their density and melt flowability, but polyethylenes with similar densities and melt flow indices may still have different physical properties, and thus be suitable for different applications.
 The key to understanding these differences is to realize that density and flowability are not fundamental properties, but rather secondary properties that emerge from the molecular architecture (microstructure) of polyethylene, as well as all other properties such as crystal morphology, mechanical, thermal, and optical properties.
The key to understanding these differences is to realize that density and flowability are not fundamental properties, but rather secondary properties that emerge from the molecular architecture (microstructure) of polyethylene, as well as all other properties such as crystal morphology, mechanical, thermal, and optical properties.
The microstructure of polyethylenes is controlled by the polymerization conditions but, more importantly, by the type of catalyst used to make the polyethylene. The catalyst is at the heart of any olefin polymerization process: properties of a polyethylene made with a good catalyst can be improved by adjusting the polymerization conditions, but the performance of a bad catalyst cannot be fixed by changing polymerization conditions.
Several coordination catalysts are used to make polyethylenes, and the ones the authors focus on in their present article fall under the category of heterogeneous Ziegler–Natta (ZN) catalysts. ZN catalysts are unique in that they comprise several types of active site, making polyethylene with different microstructural distributions. Each ZN catalyst, therefore, makes “polymer blends” with different molecular architectures, leading to polyethylenes with different product characteristics.
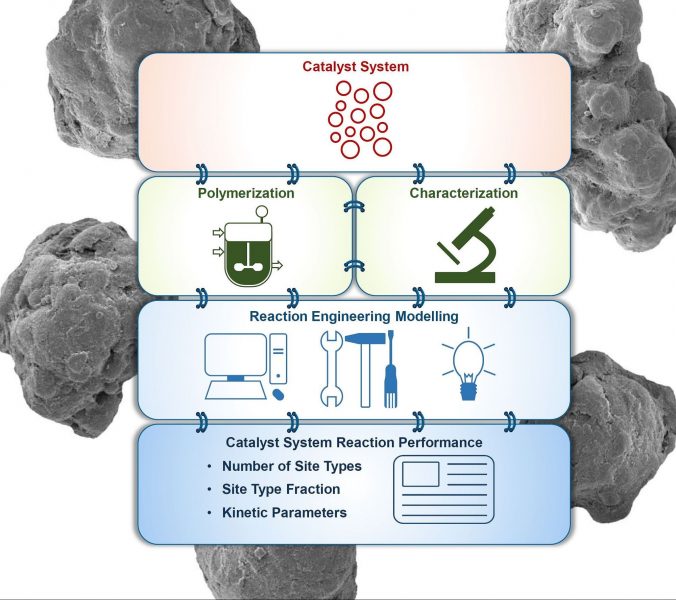
The polyolefin industry wants and needs to control the microstructure of polyethylenes made with these complex catalysts, and the best way to achieve such control is to develop mathematical models to predict the polymerization kinetics and microstructures of polyethylenes made under different polymerization conditions and catalysts.
Proposing an integrated and general methodology to develop such models is the main objective of the present article. The authors rely of the fact that everything that happens during the polymerization with a ZN catalyst is locked into the polymer’s microstructure. Therefore, by deconvoluting the molecular weight distribution (MWD) and comonomer composition distribution (CCD) of polyethylenes, and simultaneously fitting polymerization kinetic curves, they can estimate polymerization kinetic parameters for each site type on the ZN catalyst used to make a series of polyethylene samples.
With these parameters in hand, they can then predict how the catalyst would behave under different polymerization conditions, which allows to optimize and design products with different microstructures. When paired with structure-property relationships, this approach allows polyolefin producers to design their products for best performance in specific applications with a minimal number of time consuming and expensive experiments.