Catheters are medical devices with widespread uses across multiple disciplines, including urinary catheters and intravascular catheters. Depending on the function of the catheter, it may be left inside a patient’s body for an extended period, so catheters must be flexible yet strong to fulfill their purpose. Polymeric, and especially elastomeric, materials are ideal for the construction of catheters, and polyurethanes are very suitable. In addition to the necessary flexibility and strength, polyurethanes may also be adjusted to improve chemical and UV resistance, biodegradability, biocompatibility, and abrasion resistance.
Like any foreign object inside a living body, catheters are risk factors for infection. The catheters are susceptible to microbial attacks, which may result in infections, called catheter-associated infections. These catheter-associated infections are caused specifically by the formation of biofilms on the catheter surface. Biofilms, structures made up of multiple microorganisms that adhere to a surface and form a protective matrix, can cause major health problems due to the protection they offer the microorganisms against antimicrobial compounds. An ideal tactic for preventing infection is to prevent the microorganisms from forming a biofilm in the first place.
Mehul Barde and colleagues, in their paper published in the Journal of Applied Polymer Science, explored the possibility of preparing a drug-loaded polyurethane material that can be used in the manufacturing of catheters. The catheters made from this material would be able to release the drug loaded in the polymers to inhibit bacterial growth and thus prevent the formation of infection-causing biofilms. The group chose sulfathiazole as the antibacterial drug for their study because of its solubility in water at the temperature and pH of the average human body.
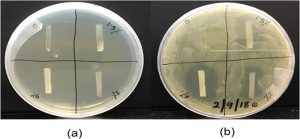
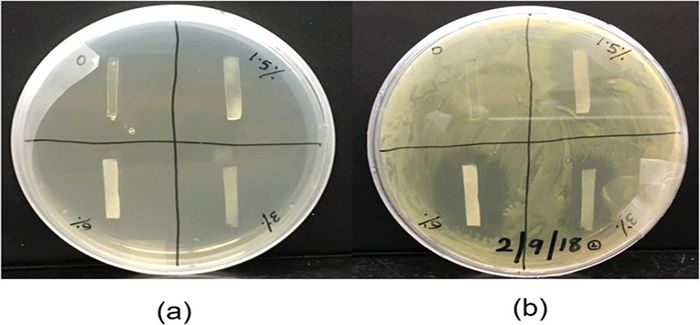
The group manufactured drug-loaded polyurethane films and assessed their thermal and mechanical properties using differential scanning calorimetry and dynamic mechanical analysis. To determine the efficiency of the released sulfathiazole, Barde and colleagues used an Escherichia coli growth inhibition test.
The image (right) shows the results of this test: (a) initially and (b) after 24 h of growth. A clear inhibition zone can be seen around the films loaded with the drug where E. coli is not able to grow, while no such zone is seen around the film with no sulfathiazole (top left quadrant of b).
Future research may examine the drug release pattern in different solutions to better predict how the catheter material would behave when in contact with body fluids such as blood or urine. Alternative antibacterial drugs with similar solubility and compatibility may also be loaded into polyurethane films for the formation of catheters.