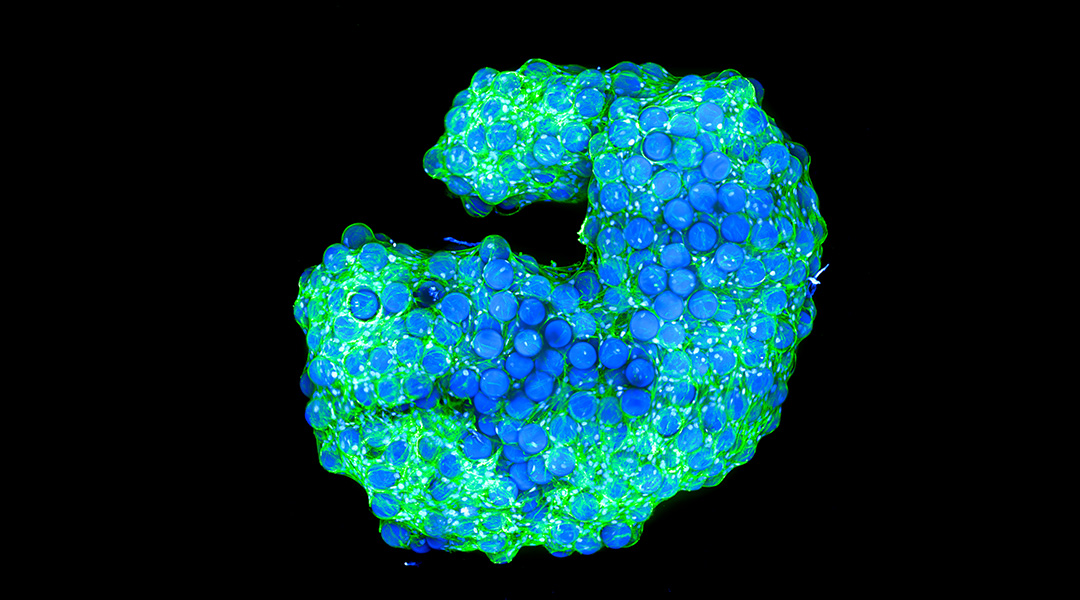
In a world where architects craft iconic structures, scientists have been able to achieve a similar feat with cells using a new type of biological scaffold that mixes cells and a material called a microgel that allows cells to grow into complex structures.
“Cells build their own microgel houses, with the microgels being the bricks and the cells the cementum gluing the bricks together,” said Daniel Günther, a doctoral candidate at the DWI – Leibniz Institute for Interactive Materials in Germany and one of the authors of the study.
The goal, he says, is to one day use these microgel constructs to rebuild natural tissue and even organs for regenerative medicine and tissue repair.
Artificial tissue made with hydrogels
Before building artificial tissues, researchers first need to build 3D cell cultures in the lab. To do so, scientists have turned to materials called hydrogels, which are webs of crosslinked polymers that come in different textures and strengths, providing an environment for cells to grow.
“Hydrogels have pores that are too small for the cells to grow. The cells have to work really hard to degrade the hydrogel network in order to grow and are still limited in their ability to form cell-cell contacts,” said Günther.
Recent advances have created microgels, which are tiny hydrogels capable of building structures where cells grow in between the microgel particles, offering more space but they do require the use of molecules known as chemical crosslinkers to link particles together, forming a framework where cells can grow.
“In these cases, cells are added to the already formed microgel construct and infiltrate it from the outside. Since the microgels are chemically glued together, the cells cannot really remodel the overall structure,” said Günther. While innovative, this approach is still limited, preventing the the growth of “natural” tissue.
In the current study, Günther and his colleagues created biological structures that do not require chemical crosslinkers to guide cell growth — in fact, the cells were found to do this on their own. This results in artificial materials that better mimic natural tissue in which cells can grow freely into complex structures determined by natural cellular mechanisms.
A serendipitous discovery
To make the new structures, the team mixed spherical microgel particles with a peptide molecule that is responsible for cell adhesion onto surfaces. Then, they added human skin cells to the mixture and, surprisingly, after 24 hours they observed the formation of a cellular scaffold that assembled without any chemical crosslinker.
Selin Bulut, a doctoral candidate at the DWI – Leibniz Insitute for Interactive Materials explained the serendipity behind this discovery. “At first, our intention was to verify the biocompatibility of the microgels that were synthesized, therefore, we simply added cells and microgels together into a well and soon we observed that the cells and microgels together formed a large construct,” she said.
This raised some interesting questions: Were there cells in every part of the scaffold? And perhaps most importantly, were the cells still alive?
Using a specialized microscope, the scientists observed cells on the surface and inside the structures, finding that they migrated to the center of the well in which they were grown, self-organizing while linking to the microgel. When observed at different time points, they found a continuous remodeling in different parts of the scaffold, demonstrating a highly dynamic nature that is similar to native tissue.
“The scaffold assembly can be described as an interplay of self-organizing cells that seek cell-cell, as well as cell-matrix interactions, which results in the simultaneous inclusion of the microgels,” said Günther.
The team also realized that by modifying the cell concentration they added to the well, the cell density in the scaffold remained almost the same. This suggests that cells use the number of microgel particles they need to build the scaffolds, and a higher amount of cells do not translate to a more compact structure.
“More cells can incorporate more microgels and fewer cells will only ‘grab’ as many microgels as they can adhere to, and therefore, the amount of microgels integrated into a scaffold is proportional to the number of cells,” said Günther.
A living scaffold
To study if cells survived inside the scaffold, the team performed an experiment that identifies live or dead cells in different colors and observed that after seven days, the cells in the scaffold were still alive. This confirms that these microgels allow for oxygen and nutrient penetration required to keep the cells alive in a 3D architecture — just as in natural tissues and organs.
“This is an efficient method to generate a tunable supporting scaffold based on microgels without the need for additional chemicals and the necessity of pre-engineered pores, where the number of microgels as well as the microgel’s properties play a crucial role for the resulting scaffold geometry,” said Bulut.
“Future applications mainly include in vitro applications such as disease modeling and drug testing as the formation of such scaffolds could be easily upscaled for high-throughput screenings,” added Günther.
“The facile scaffold formation without the need for additional chemical modifications makes this system interesting for clinical applications,” he continued. “The cell-induced scaffolds could also be applied in regenerative medicine in the future, where the scaffold material can be resorbed after tissue regeneration.”
This technology is not limited to the specific microgel particles they used nor to the human skin cells they tested. “There are various materials with distinctive properties such as different mechanical and surface properties, and degradation kinetics, which can be tailored for the specific application area,” added Bulut.
Reference: Selin Bulut and Daniel Günther, et. al., Cellular Architects at Work: Cells Building their Own Microgel Houses, Advanced Healthcare Materials (2023). DOI: 10.1002/adhm.202302957
Feature image: Cell-induced interlinked scaffold built in a well. In green, cell cytoskeletons; in blue, cell nuclei. Credit: Selin Bulut and Daniel Günther, DWI –Leibniz Institute for Interactive Materials
ᐧ