The term ‘theranostic’ is coined by blending ‘therapeutic’ and ‘diagnostic’, forming a portmanteau word that describes a single agent that combines both diagnostic and therapeutic capabilities under the the emerging personalized medicine paradigm. Nanomedicine proves to be a convenient approach to theranostics as the properties of nano-sized materials can be tailored and optimized for targeted therapeutic and/or diagnostic purposes. A versatile class of such materials are gold nanoparticles (AuNPs), which have already been widely studied in many fields of medical research, such as drug delivery, tumor detection, and gene therapy.
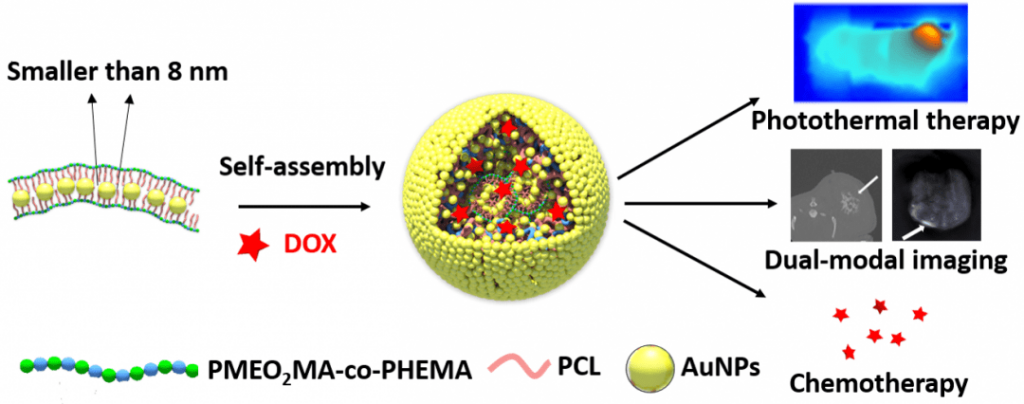
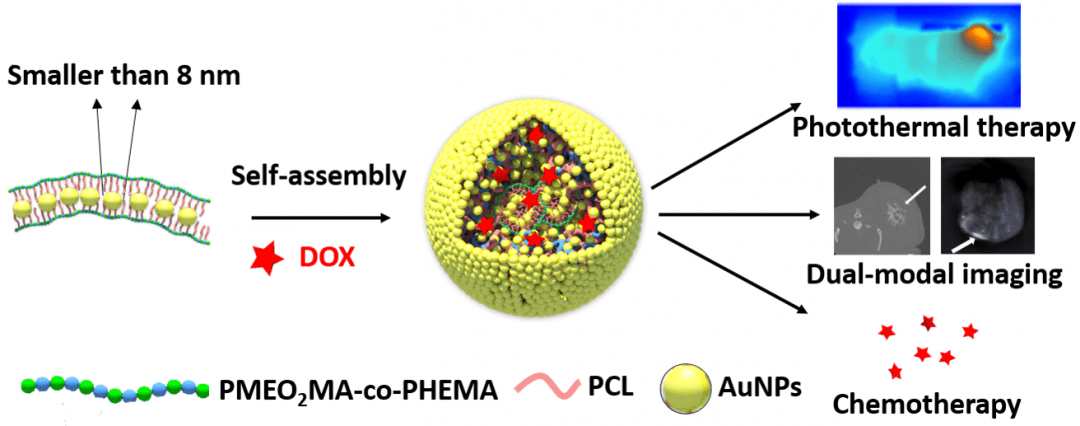
In particular, the tunable surface plasmon resonance (SPR) of colloidal gold is ideal for photothermal therapy (PTT) and photoacoustic tomography (PAT), while the high mass attenuation coefficient provides an advantage in X-ray computed tomography (CT) imaging. However, colloidal gold is also a double-edged sword when it comes to biomedical applications because it shows size-dependent toxicity. Smaller AuNPs, say, those with core sizes under 8 nm, are desirable due to low toxicity, but in the meanwhile, when the particle size reduces, it becomes much more difficult to generate SPR in the longer wavelength, especially the near infrared (NIR) region, an ideal spectrum window that shows both good biocompatibility and excellent dermal transmission.
This dilemma has now been circumvented by Xin Zhang and co-workers at Institute of Process Engineering, Chinese Academy of Science (Beijing, China), using an amphiphilic polymer as a template to assemble small AuNPs into gold nano chains, which can then further aggregate into gold micelles a few hundred nanometers in size with enhanced NIR absorption. Such design is based on the fact that gold nanorods show stronger SPR than AuNPs, which also holds true in the case of linearly connected and discrete AuNPs.
The researchers first synthesize the comb-like polymer host using reversible addition-fragmentation chain transfer (RAFT) and ring opening (ROP) polymerization in two consecutive steps, followed by the side chain decoration with mercapto groups in order to increase affinity to gold. In the presence of the polymer host, 6 nm AuNPs are prepared from the reduction of chloroauric acid by sodium borohydride, embedded as chain-like assemblies. While the SPR peak of polymer-supported gold chains shows significant red shift from discreet AuNPs, their aggregation into gold micelles further shifts the peak to the NIR region. As the authors note, “from the perspective of topology, one-dimensional chains are better choices than discrete AuNPs to obtain solid micelles”.
Through standard cell toxicity assays, the researchers demonstrate that the gold nanomicelles all highly compatible with three different types of cells. These gold nanomicelles are then tested as an in vitro PTT agent, and upon irradiation of 808 nm laser, they can induce highly selective and localized apoptosis of Michigan Cancer Foundation-7 (MCF-7) cells. In addition, the micelles can be used to load hydrophobic chemotherapy agents such as doxorubicin (DOX) for drug delivery. On the other hand, in vivo mice CT and PAT imaging experiments reveal that these gold micelles are highly effective contrast agents. More importantly, these functional micelles will not accumulate in the body as they can easily dissemble into non-toxic AuNPs and polymers, which ultimately receive kidney clearance.
With this multimodal theranostic material in hand, the authors are planning to further reduce the size of AuNPs as well as their assemblies, because “the smaller size of AuNPs will benefit the rapid clearance of AuNPs via kidney clearance…, and make it possible for intravenous injection which is a more common route for drug administration in clinical medicine”, as envisioned by the authors. Another effort to improve this system will focus on developing “smart” environment-responsive gold chains “that can be induced to self-assembly only in the special physiological environments”, because, for example, large nanomaterials are unable to cross the so-called blood brain barrier, limiting their applications in treating brain diseases. The authors hope shorter gold chains can cross this barrier, and “then aggregate into nanomicelles in lesion areas”, a promising strategy for certain malignant brain diseases, including brain glioma.