Searching out news ways to efficiently build molecules is a challenging yet imperative task in drug discovery. Rings in particular play an important role in many active ingredients, but once they’re formed, they can be difficult to modify.
“Ring-based molecules are particularly important in many fields because the ring structures provide special rigidity and orientation in space, as well as special electronic properties,” explained Frank Glorius, professor of chemistry at the University of Münster. “For this reason, most functional molecules in the pharmaceutical and agrochemical industries, as well as in other fields, contain ring structures.”
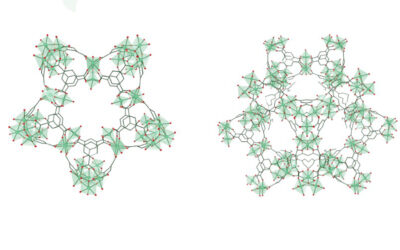
Glorius and a group of collaborating scientists have recently reported an unprecedented approach that allows them to “edit” a single atom within a molecular skeleton. “Very few single-carbon insertion reactions have been developed in the last hundred years,” he said. “More importantly, these reactions usually achieve a single functionalized carbon insertion, so the carbon contains a halide, an ester, or an aromatic group. In contrast, our transformation can achieve various functionalized carbon atom insertions by photocatalysis.”
Their new reaction allows them to incorporate a single carbon atom into the framework of carbon-based rings to adjust the ring size from five to six-membered rings. In the past, this was only possible with nitrogen atoms.
“By contrast, inserting a single carbon atom into an all-carbon ring is an enormous challenge,” said Fu-Peng Wu from the University of Münster and the study’s first author in a statement. “The carbon reagent needs to be compatible with various functional groups, which determine the chemical properties of the molecule. In addition, the compound should be stable, easy to handle, and susceptible to uncomplicated activation.”
The reaction makes use of a photocatalyst, which can alter the rate of a chemical reaction by absorbing light. When exposed to light, photocatalysts undergo a photochemical process that results in the initiation or acceleration of a specific chemical reaction — in this case, the formation of a carbyne radical that contains three unpaired electrons and facilitates the insertion of carbon atoms, even when they bear a variety of other functional groups.
“We were able to elucidate the reaction mechanism in collaboration with the group of Prof. Osvaldo Gutierrez — we would not have been possible to do this without them!” said Glorius. “The key to success is the use of the special carbon reagent and its activation with a catalyst and visible light. In the further course of the reaction, parts of the molecule then rearrange via the intermediate formation of strained three-membered rings.”
This, say the team, provides opportunities in skeletal editing that were not previously possible. “For one carbon is the premier element of organic molecules, second the attached groups can be varied greatly,” added Glorius.
While this will open doors in synthesis, allowing chemists to explore new and varied structures, the approach is limited to five-membered rings, and does not get work on rings that contain atoms other than carbon, such as nitrogen or oxygen, which are also important in pharmaceuticals.
“Numerous research groups are currently working in the fascinating field of skeletal editing,” said Glorius. “Surprisingly, almost magical transformations can be expected. There are so many different ring structures and even more challenges out there.”
Reference: Osvaldo Gutierrez, Frank Glorius, et al., Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion, Nature (2024). DOI: 10.1038/s41929-023-01089-x
Feature image credit: © Uni MS – Fu-Peng Wu