Protein phosphorylation is an important post-translational modification that changes the conformation and activity of proteins. Phosphorylation is reversible and so phosphorylation/dephosphorylation can be viewed as an “on-off switch” regulating cellular processes.
Protein phosphorylation is an important post-translational modification that changes the conformation and activity of proteins. Phosphorylation is reversible and so phosphorylation/dephosphorylation can be viewed as an “on-off switch” regulating cellular processes.
Aberrant phosphorylation is implicated in a number of serious diseases including cancer or neurodegenerative diseases such as Alzheimer’s and Parkinson’s. The analysis of protein phosphorylation at the proteome level is crucial for our understanding of physiological and pathological processes, and it has potential in drug development and as a diagnostic tool.
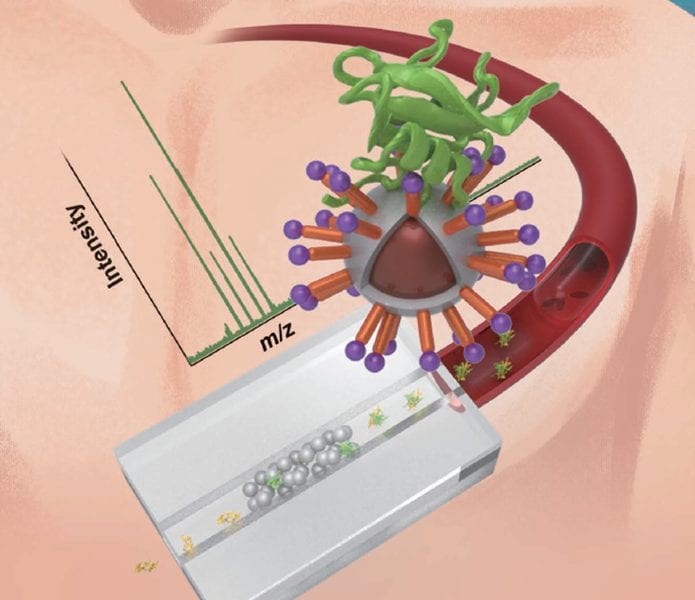
However, the analysis of phosphorylation in complex biological samples is a challenging task due to the low abundance of phosphorylated proteins. Before analysis by mass spectrometry, the digested samples have to be therefore efficiently enriched in phosphopeptides. This is typically performed using ion exchange chromatography or affinity capture chromatography such as metal oxide affinity chromatography. Researchers from Jilin University, China, implemented the latter principle in a microchip coupled with MALDI-TOF MS. Indium oxide-functionalized magnetic nanoparticles were synthesized and used as solid phase extraction adsorbent on a chip, held with the help of an external magnetic field.
The performance was tested not only on standard protein digests of β-casein and bovine serum albumin and ovalbumin, but also on “real”, complex samples including milk and human serum. The magnetic SPE-MALDI-TOF MS system with In2O3-coated Fe3O4 magnetic nanoparticles showed highly selective, reproducibility, could be reused several times and enabled a very low phosphopeptide detection limit of 4 fmol.