Glioma is one of the most malignant central nervous system cancers and is rarely curable. The current treatment options are limited to surgery in combination with radiation therapy and/or chemotherapy. Due to the location of the tumor, either in the brain or spine, it is not surprising that these treatments carry significant risks and often result in severe side-effects.
Targeted drug delivery for cancer treatment promises reduced side-effects and higher efficiency by using nanocarriers that deliver the drug directly to the tumor site. Targeted therapy has been successfully implemented in the treatment of many types of cancer such as breast, colon, lung and others. For the treatment of brain cancer however, this type of therapy is problematic, mainly due to the virtual impermeability of the Blood-Brain-Barrier (BBB) which prevents more than 98% of small molecules administered to the patient from reaching the brain.
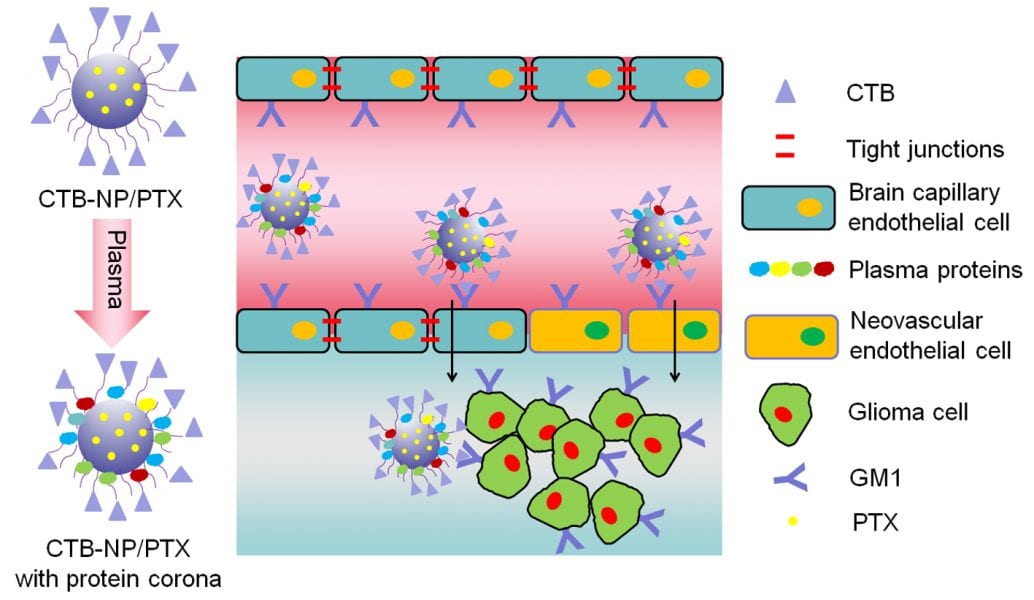
To tackle this issue, researchers have used two or more ligands to modify the nanocarriers in order to escape the strict BBB selection, by preventing their deactivation by the digestive enzymes. However, this type of modification has several drawbacks. First, it makes the preparation process very complicated. Second, it is unknown whether the different ligands interfere with each other, thus reducing the overall activity. Also, in the recent years, proteins (and protein-like molecules) have been widely used as targeting ligands. However, when these molecules are modified on the surface of nanocarriers, they will thoroughly interact with plasma proteins (as well as enzymes). It is very common that the plasma proteins coat the nanocarrier surface or that the enzymes digest the targeting molecules, thus inducing the deactivation of the ligands. Therefore, the goal is to achieve multifunctional glioma-targeted drug delivery and to ensure that the targeting molecules are still bioactive in vivo.
In a recently published article in Advanced Healtcare Materials, scientists from China demonstrate a multifunctional targeting of intracranial glioma inspired by the intoxication mechanism of the Cholera toxin. The PLGA nanoparticles modified with the toxin (CTB-NPs) are shown to successfully penetrate the BBB in-vitro and effectively target glioma cells and neovasculature. In addition, the stability of cholera toxin subunit B in plasma, as well as the protein components of the protein corona (plasma proteins coating on the nanocarrier surface), are thoroughly studied in vivo, and the results indicate that the attached targeting ligand is still active.
Furthermore, when loaded with the chemotherapeutic drug, Paclitaxel, these targeting nanoparticles (CTB-NP/PTX) induce cell death of glioma cells and remove neovasulature in mice with glioma, resulting in prolonged survival.
The overreaching impact of this study is providing a single protein that can achieve multifunctional targeting of glioma for drug delivery. Future studies will focus on the safety and efficacy, such as immunogenicity assessment, in larger animals and humans.