Waste products from industrial processes often contain chemicals that are damaging to the environment and to human health. Chemicals containing hexavalent chromium (Cr(VI)), as produced in leather-tanning, paint-making, and steel manufacturing industries, are particularly problematic. Cr(VI) is highly toxic to living organisms, as well as carcinogenic, and its high water solubility means that it quickly spreads in the environment. Due to this, reducing the amount of Cr(VI) released into the environment is a significant priority for waste treatment. On the other hand, trivalent chromium (Cr(III)) is environmentally benign, and not particularly mobile in the environment. As such, reduction of Cr(III) is a good strategy for rendering Cr(VI)-containing waste-water, and can be achieved by photocatalysis using semi-conductors, such as the titanium oxide based P25. However, the wide bandgap of TiO2 means that the reduction reaction can only be catalyzed by high-energy UV radiation, severely limiting the reaction rate. Other, visible-light active photocatalysts have been found, but these are not generally stable, and the catalysts themselves are often toxic. 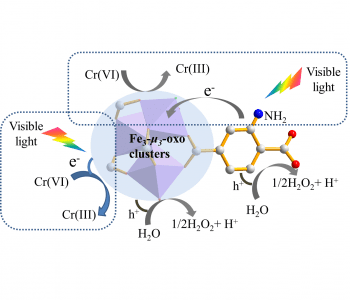
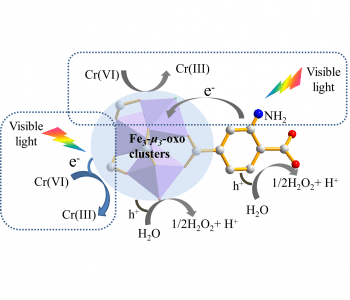
Researchers in Japan have developed a much more efficient photocatalyst for the reduction of Cr(VI) to Cr(III), consisting of an iron-based metal–organic framework, functionalized with amine groups. Metal–organic frameworks are hybrid materials, made up of metal–oxo clusters and organic ligands in a porous network. The porosity means that the catalysis reaction can occur over a large surface area, and tuning of the band gap of the MOF can be achieved by modification of the organic or inorganic components during synthesis, allowing for visible-light-activated photocatalysis. The introduction of an amine functional group increases the photocatalytic activity of the Cr(VI) reduction reaction in several iron-based MOFs, suggesting that this could be an effective general method obtaining iron-based MOF photocatalysts with improved performance. The photocatalysts were created via microwave-assisted solvothermal synthesis, and were found to be stable, and easily reusable after filtration and washing.
The mechanisms behind the improvement in photocatalytic activity were investigated by photoluminescence, electron spin resonance, and transient photocurrent studies. Both the iron–oxo cluster and the amine groups are excited by visible light. The electrons generated from the excitaction of the amine group are transferred to the iron–oxo cluster, where the reduction of Cr(VI) to Cr(III) takes place. The amine group thus leads to a larger number of electrons available for the reduction reaction, with the amine-generated electrons having a longer lifetime, due to the electron–hole pairs being separated in the transfer to the iron–oxo cluster. This dual-excitation mechanism leads to much greater efficiency or the reduction reaction with increased concentration of the amine-functional groups.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.