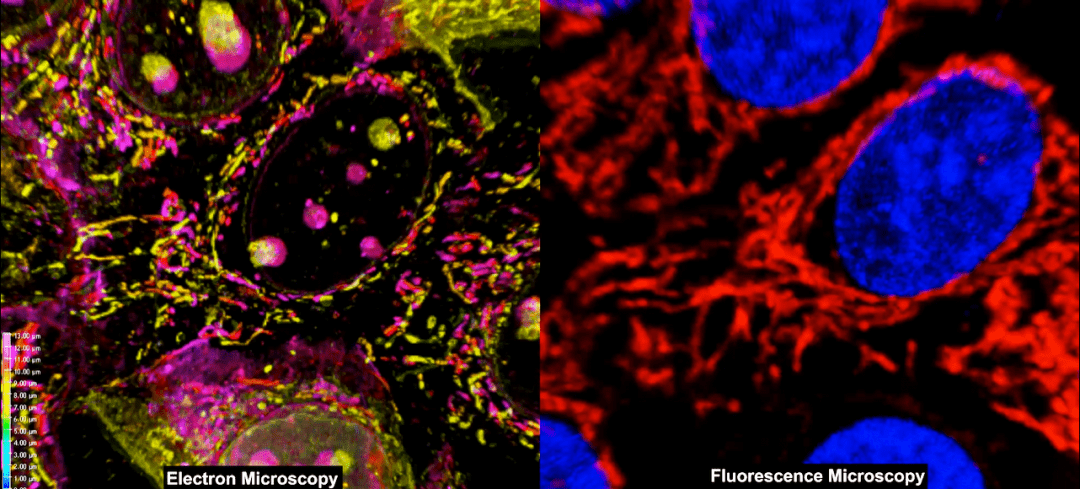
Fluorescence microscopy (FM) has come a long way. Biologists have gone from being able to view cells under the microscope to now imaging the dynamics of molecules within cells. Electron microscopy (EM) goes a step further and provides angstrom-level resolution. These advanced forms of microscopy have allowed characterization of interactions at a cellular level. Combining the high-throughput and large scale analysis of FM with the enhanced resolution capabilities of EM can allow imaging of cellular interactions and mechanisms without being limited by resolution. A new method described in the April 2017 Supplement of Current Protocols in Cytometry does just that.
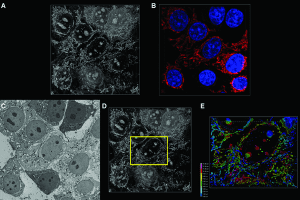
Imaging of cell culture specimen
Simon Watkin’s group at University of Pittsburgh details a protocol for a novel method to correlate the results of fluorescence confocal (FM-confocal) and scanning electron microscopy (SEM) to elucidate the 3D structure of cells and tissue specimens.
Existing methods such as cryo-TEM tomography or 3View used for for evaluating cellular structural features, require specialized equipment and are highly sophisticated. The protocol described by Watkins et al. avoids extensive and laborious sample preparation or sophisticated electron microscopy setups and provides a straightforward way of correlating optical microscopy and electron microscopy data.
Video demonstration of 3D rendering
The Anticipated Results section contains a video (see adjoining screenshots) that demonstrates image acquisition and analysis with this protocol so users know what to expect. Users will find the Critical Parameters section particularly helpful for paying attention to aspects that can affect outcome and help avoid pitfalls from the get go.
This protocol is a novel, straightforward and thorough approach to generate high fidelity and high resolution imaging data of cellular arrangements and interaction mechanisms, without the need for extensive and laborious sample preparation or sophisticated electron microscopy setups.
For established and seminal methods in cytometry, please visit Current Protocols in Cytometry.