Over half of the diagnostic imaging exams performed worldwide are done using X-rays, which remains vitally important for diagnosing diseases with techniques including radiography, mammography, and computed tomography (CT). In order to help with diagnoses, many patients are injected with contrast materials, which can absorb X-rays, to distinguish normal from abnormal conditions or brighten certain organs. Up to now, iodine molecules and barium sulfate suspensions have been the only X-ray contrast agents used in clinical medicine. But there is increasing concern over their safety in the body and suitability for newly developed X-ray imaging techniques.
In a recent study published in WIREs Nanomedicine and Nanobiotechnology, Professor David Cormode and colleagues, who work at the forefront of nanomedicine research and X-ray based technology developments, explore advanced X-ray imaging system designs and new types of X-ray contrast agents and their relevant biomedical applications that have been reported in recent years.
Current X-ray imaging is based on simple “gray values” to determine changes in anatomy and pathology on a larger scale. But this offers little functional information about the subtle differences on the biomolecular level, which is very important for early disease detection.
The invention of spectral photon-counting CT (SPCCT) is a notable advancement in CT technology. The ability to count and discriminate individual X-ray photons results in improved image quality that enables the visualization of small structures like blood vessels. With SPCCT, one can also gain information about the compositions of an object on the elemental level, which is useful in differentiating tissue materials.
Similarly, dual-energy mammography (DEM), which is clinically approved, uses two different X-ray energies to enhance the signal from the contrast agent and provide anatomical data of diagnostic quality. The higher diagnostic sensitivity of DEM compared to conventional mammography significantly improves breast cancer screening for women with dense breasts (who are also at increased risk for the disease). Recently, information about breast tissue compositions, such as in breast density, can be obtained by photon-counting mammography.
Iodine and barium do not produce optimal contrast in SPCCT and DEM, therefore this has sparked interest in alternative elements, such as silver, gold, tantalum, bismuth, and gadolinium.
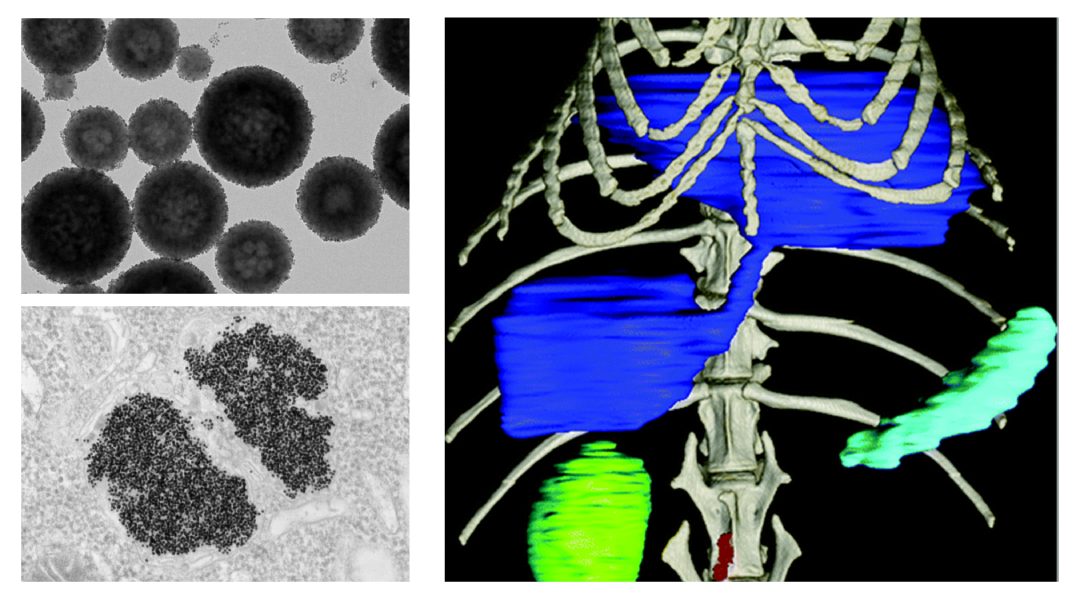
Much of the research on novel X-ray contrast agents in the past two decades has been devoted to nanoparticle formulations with chemical compositions based on heavy metal elements. Nanoparticles are very small structures that range between 1 and 1000 nm in size and may contain up to millions of contrast-generating atoms.
Nanoparticles that are smaller than 5 nm in size with neutrally charged coatings can undergo rapid renal clearance from the body, which is an essential criterion for clinical translation. This has been demonstrated with proposed CT contrast agents using gold nanoparticles coated with glutathione, or tantalum oxide nanoparticles coated with polymers that contain both positive and negative charges.
On the contrary, those that are larger in size and coated with long polymer chains like polyethylene glycol (PEG), which are frequently used in current drug delivery systems, can bypass renal clearance and prolong circulation times for longer lasting contrast and greater chance to enter disease sites. For example, PEG-capped gold silver alloy nanoparticles are long circulating agents that accumulate in breast tumors and make them more visible in both DEM and CT.
Furthermore, the nanoparticle surface can be further decorated with targeting moieties to increase delivery to the target sites, thereby improving diagnostic and therapeutic outcomes. For example, nanoparticle CT contrast agents based on platinum were conjugated with trastuzumab, a chemotherapy drug, to image breast cancer that highly expresses HER2 targets. In addition, SPCCT imaging with bismuth or ytterbium nanoparticles targeted with fibrin-specific antibodies can distinguish thrombus from plaque calcium deposits.
X-ray nanoparticle contrast agents have also opened the door to a new era in cell tracking. Malignant brain cells or immune cells are often labeled with gold nanoparticles and monitored via CT imaging to learn about the dynamics of a growing tumor or study atherosclerosis progression. Such an imaging approach offers ways to better understand cellular behavior and trafficking patterns, which are important to the development of cell-based therapies.
Indeed, X-ray nanoparticle contrast agents can be specifically designed to yield desired properties for relevant imaging and therapeutic applications. This field has seen tremendous progress in the last few decades, but more developments will be made to expand the functions of current agents and to achieve clinical approval for use in patient care.
Written by: Jessica C. Hsu, Lenitza M. Nieves, Oshra Betzer, Tamar Sadan, Peter B. Noël, Rachela Popovtzer, and David P. Cormode
Reference: J.C. Hsu, et al. ‘Nanoparticle contrast agents for X‐ray imaging applications.’ WIREs Nanomedicine and Nanobiotechnology (2020). DOI: 10.1002/wnan.1642