Poly(acrylamide) and its copolymers are important in a variety of commercial applications including cosmetics, pharmaceuticals, water treatment, mining, oil sands, textiles, and paper processing. The materials are produced and used in aqueous solution, as both the starting monomer and the resulting polymer are soluble in water. Understanding of the kinetics of these radical polymerization systems has lagged behind that of organic-based systems because of sampling and characterization difficulties and due to their unusual kinetic features. For example, while the rate of polymerization is expected to be first order with respect to monomer concentration, studies with acrylamide (AAm) report a reaction order between 1.2 and 1.5.
The application of specialized pulsed-laser kinetic techniques is starting to shed light on this unusual behavior. Specifically, the recent study by Kattner and Buback (University of Göttingen, Germany) has shown that intramolecular chain transfer, a reaction that leads to branched structure and is commonly referred to as “backbiting”, occurs in the system (Macromol. Rapid Commun. 2015, 36, 2186). With this new knowledge comes the opportunity to provide an improved mechanistic description of acrylamide polymerization under industrially-relevant conditions. The research groups of Robin Hutchinson (Queen’s University, Kingston, Canada) and Igor Lacík (Polymer Institute of the Slovak Academy of Sciences, Bratislava, Slovakia) have combined to develop a complete model of acrylamide polymerization, verified using available literature data as well as new experimental results obtained using an in-situ NMR technique to measure monomer conversion profiles and size-exclusion chromatography to characterize the resulting polymer.
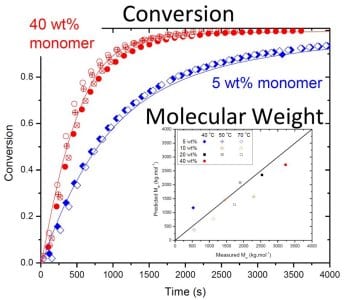
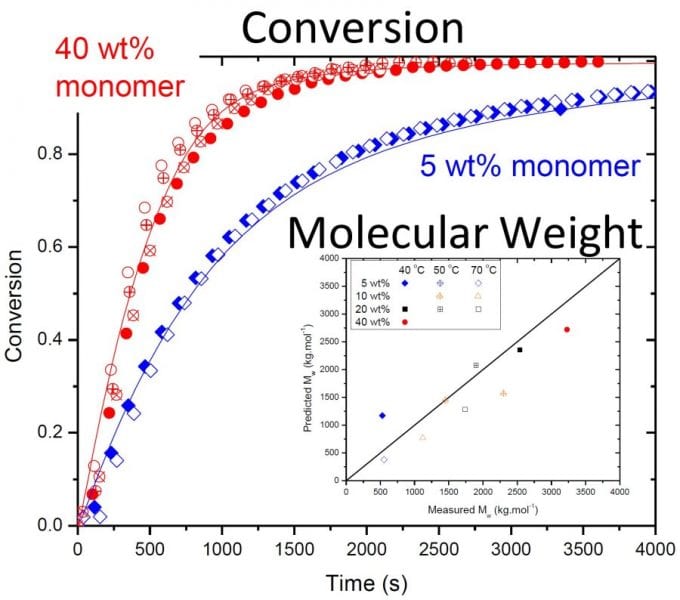 The work shows that although backbiting does not result in measurable branching in the poly(acrylamide) formed, the mechanism is required to explain the reduced rate of monomer conversion observed in batch polymerizations when initial AAm concentration is lowered from 40 to 5 wt%, as shown in the figure. The new model is able to fully capture the effect of the kinetic complexities of the system on polymerization rates and polymer molar masses over a wide range of operating conditions. The understanding gained through this model development for the homopolymerization of acrylamide can be combined with that recently published for acrylic acid (Wittenberg et al., Macromol. React. Eng. 2016, 10, 95) to form a solid basis for the modeling of the copolymerization of these two important monomers.
The work shows that although backbiting does not result in measurable branching in the poly(acrylamide) formed, the mechanism is required to explain the reduced rate of monomer conversion observed in batch polymerizations when initial AAm concentration is lowered from 40 to 5 wt%, as shown in the figure. The new model is able to fully capture the effect of the kinetic complexities of the system on polymerization rates and polymer molar masses over a wide range of operating conditions. The understanding gained through this model development for the homopolymerization of acrylamide can be combined with that recently published for acrylic acid (Wittenberg et al., Macromol. React. Eng. 2016, 10, 95) to form a solid basis for the modeling of the copolymerization of these two important monomers.