The CRISPR–Cas9 bacterial immunity system was discovered and engineered for use as an efficient genome editing tool in a range of organisms and applications. The system can correct disease-causing mutations in a flexible and affordable manner. However, its efficiency is still limited in difficult-to-transfect cells such as primary human T cells. Due to this, improved tools are highly needed to efficiently target and edit genes to modulate T-cell function and correct disease associated mutations.
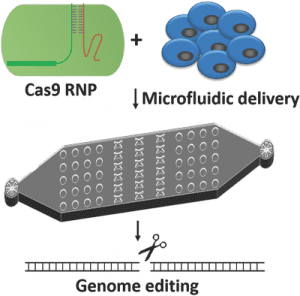
Microfluidic cell deformation-based method.
Researchers from the Qin lab have developed a microfluidic cell deformation delivery method that uses physical constriction to deform and shear cells for delivery. Rapid mechanical deformation of cells can produce membrane disruptions or holes that allow passive diffusion of materials into the cytosol. By targeting PD-1(PDCD-1), a validated target for tumor immunotherapy, knock-in genome modifications were achieved in human primary T cells. This delivery method has the potential to facilitate Cas9 RNP-directed genome editing and support the utilization of gene therapy as a human therapeutics tool.

















