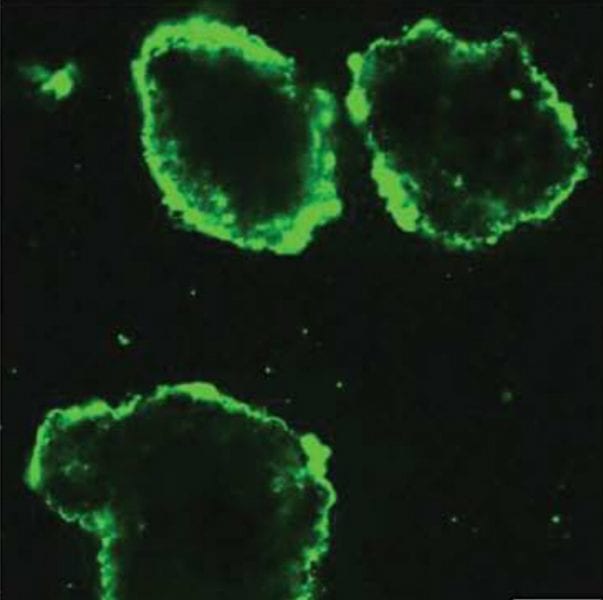
Diabetes is a chronic autoimmune disease that is associated with destruction of insulin-secreting pancreatic β-cells affecting 23.6 million people in the US alone. One of the most promising paths towards a diabetes cure is transplantation of pancreatic islets (insulin-producing cell clusters). The procedure involves transferring healthy islets into the diabetic recipient’s portal vein in the liver. The transplanted cells begin producing insulin, restoring the patient’s immune system without the need for insulin injections. Despite the significant potential of this treatment, its clinical application is limited due to islet instability during isolation and culturing, and also because of immunosuppression in the patient’s body, resulting in transplant rejection.
To overcome these complications, an American research team have developed an approach relying on modification of living pancreatic islets with ultrathin cytoprotective coatings with immunomodulatory capabilities. These coatings are deposited on the surfaces of mammalian islets via layer-by-layer (LbL) hydrogen-bonded assembly of cytocompatible polymers. The coating is conformal, stable, non-immunogenic, allows for coupling with functional molecules, and capable of prolonging islet viability and function. Indeed, coated islets show a significant increase in insulin secretion as compared to uncoated islets. In addition, the coating material demonstrates immunomodulatory effects by suppressing pro-inflammatory cytokine synthesis. Both features are critical for islet transplantation and opens new opportunities for advanced multifunctional materials for a cell-based transplantation therapy.