Every time you eat a meal, the food you chew travels down your esophagus and into your stomach where gastric juice makes a first attempt at digestion. But the vast majority of nutrients are absorbed by the small intestine, which carries the semi-liquid mass of partly digested food on a five meter roller coaster ride, leading to the large intestine.
Along the way, the mass that used to be your food is squeezed and pushed around in thousands of different ways. It is broken down into even smaller pieces, which are mixed among themselves and pressed against the walls of the intestine in order for the relevant nutrients to be absorbed, all the while being propelled along its snaky path by an array of muscles, pacemakers, and neurons.
However, in some patients, this journey is cut short. A condition known as short bowel syndrome (SBS) affects only a handful of people per million but takes a huge toll on their lives. In this syndrome, a portion of the small intestine is lost or non-existent, where the remaining part cannot absorb enough nutrients to sustain life.
Patients must be fed through an IV, but the almost constant hospitalization, possible side effects, and up to 50% mortality rates are far from ideal. Transplants, the main alternative, come with long waiting lists and high risk of rejection.
An artificial intestine
This is why researchers dream of a better solution: engineering an artificial intestine that can make up for the bit that SBS patients lack. While that dream is probably still decades away, their paper published in Advanced Materials takes a decisive step towards achieving it. The study develops a patch of intestinal muscle that contracts and is able to break down some artificial materials simulating partially digested food.
“Most of the effort has been devoted to creating the epithelial [i.e., absorption] layer […] however, not a lot had been done in developing the muscle layer. And the muscle layer is very important, because if there is no motion, you can’t mix the food and you can’t push it from the small intestine to the large intestine,” said Qianqian Wang, researcher at the University of Stanford and lead author of the paper.
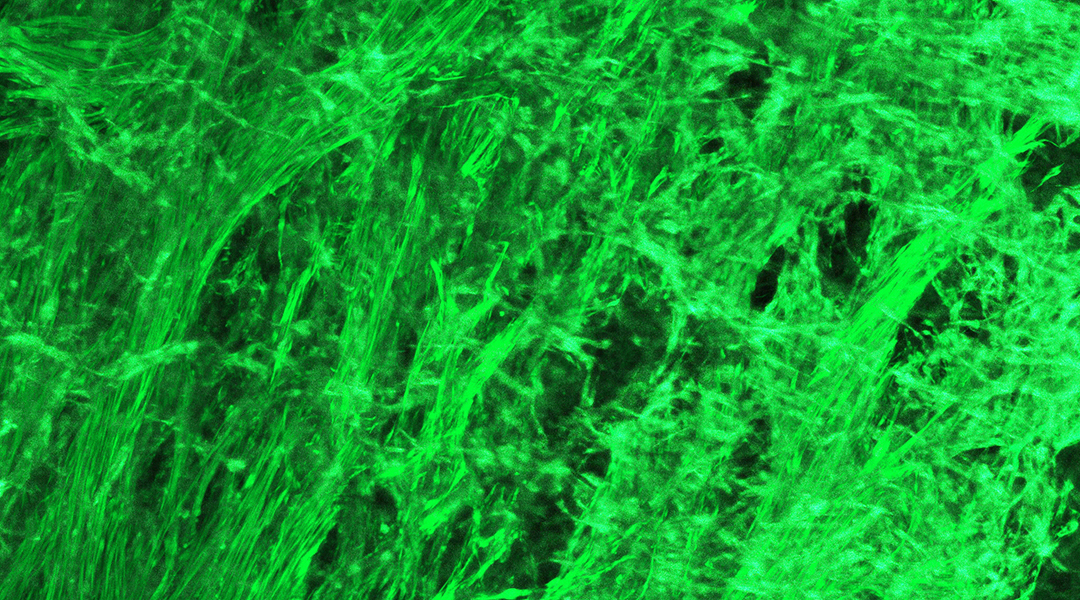
Wang and her colleagues placed several different kinds of cells of the kinds that make up the muscle layer of a real intestine in a medium that would allow them to thrive, and then built them a “scaffold” out of gelatin so they would form the desired structure. Their hope was that, after letting the cells grow, the resulting tissue would be able to contract.
Better than expected
To their surprise, their engineered tissue displayed much finer skills. They witnessed three different contraction patterns, which they dubbed clamshell, ocean-wave, and jellyfish. “When you hold your well plate [where the tissue cells were growing] and by your eyes you can see them contracting in the plate — it’s super cool,” said Wang.
Encouraged by these positive results, they gave their tissue an artificial substance similar to partially digested food and were amazed to find it could break it down like an intestine would.
Previous attempts had not been anywhere near as successful at obtaining a tissue that emulated the contractions of an intestine. Some proposals used a kind of plastic as the scaffold, but Wang and her co-authors thought gelatin would be more similar to the real tissue. “We tried to mimic the real thing, and I think it paid off,” she said, still surprised at the performance of her experiment.
Next steps
The biggest difference between an actual intestine muscle layer and the muscle patch they developed, Wang explained, is the immune population. Having a strong immune system in the intestine is crucial to prevent food entering the body from causing us harm, so the team is working on improving this aspect.
Mechanical properties, like elasticity or suppleness, also need tweaking to make them as similar as possible to the real tissue. This might be a way to go from the three motor patterns they have obtained so far to the thousands that an actual intestine has.
Plus, cells also feel the mechanical aspects, explained María C. Serrano, researcher at the CSIC Materials Science Institute of Madrid. “It was overlooked until recently, but if [a tissue] is not mechanically adequate, it ends up being rejected” by the body.
The next step in the windy road ahead will be to combine the muscle layer with an epithelial layer, for which proposals already exist. Wang thinks that one can build a scaffold to accommodate both layers, and hopes to design experiments to test this idea. One of the main challenges going forward is understanding the small intestine in even more detail than science currently does, so bioengineers can have a more precise picture of what they’re aiming for.
“If we are lucky, first we may be able to combine the epithelial layer with the muscle layer in five years,” said Wang, “and then in another ten years we may be able to actually create the real tissue.”
Reference: Qianqian Wang, et al., An Engineered Living Intestinal Muscle Patch Produces Macroscopic Contractions that can Mix and Break Down Artificial Intestinal Contents, Advanced Materials (2023). DOI: 10.1002/adma.202207255