In a plasma, monomers can react and the resulting polymers can form thin films, when deposited on a substrate. The properties of the resulting films depend on the monomers used and they typically have a relatively complex structure: this diverse range of functionalities, makes them ideal for use in industrial and research applications such as hydrophilic films, non-fouling surfaces, water purification, and biomaterial coatings.
Various techniques have been used to characterize the chemical and physical properties of plasma polymer thin films. Most techniques have been used to probe films post production and in their dry state but there is an increasing number of applications that require stable plasma polymer films in aqueous conditions, for example, to make novel biomaterials. However, while dry films can be easily characterized, a lack of understanding of the internal structure and behavior of the films in hydrated conditions limits scale up of the technology for commercial products: it is evident that a multi-technique approach is necessary to probe the complex environment plasma polymer films.
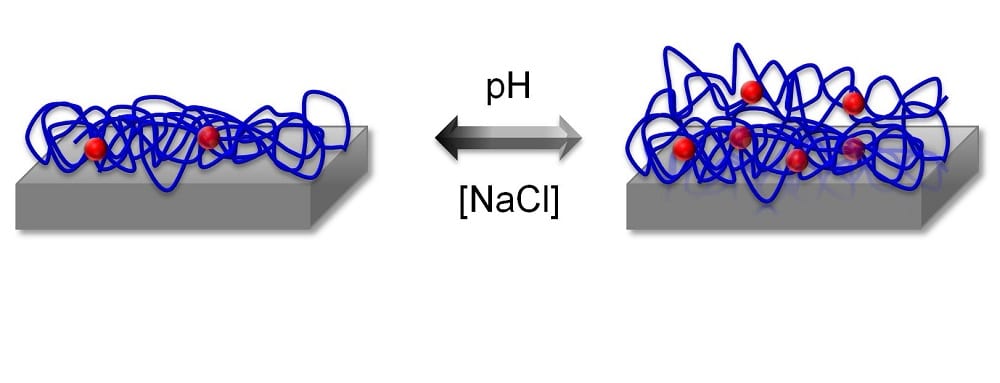
In a study published by Plasma Processes and Polymers, two complementary techniques, quartz crystal microbalance with dissipation techniques (QCM-D) and electrochemical impedance spectroscopy(EIS), have been used to monitor the changes in the film in aqueous conditions, when the pH and the ionic strength of the solution have been changed. QCM-D can give information about film thickness and its viscoelastic properties, whereas EIS can be used to probe the dielectric properties of such a film. By combining results from both techniques, a swelling of the film has been observed, and under certain conditions, it seems that the top interface of the film exhibits a different behavior than the bulk of the film. The effect of ionic strength and pH on the structure of hydrated plasma polymerized films of allylamine (ppAAm) and acrylic acid (ppAAc) has been analyzed in situ using quartz crystal microbalance with dissipation techniques, electrochemical impedance spectroscopy, ellipsomtery, and X-ray photoelectron spectroscopy. Both materials showed a salt concentration and pH dependent uptake and release of water and ions. Depending on the type of monomer used, the effects showed reversible or non-reversible behavior. The investigation of the electrochemical properties of the film further revealed a non-homogeneous structure, especially in the case of ppAAc films, with regions of higher and lower cross-linking density. The use of complimentary techniques to characterize the films in situ allowed for a deeper understanding of processes happening inside the plasma polymerized films, which can help to optimize film preparation conditions for selected applications.